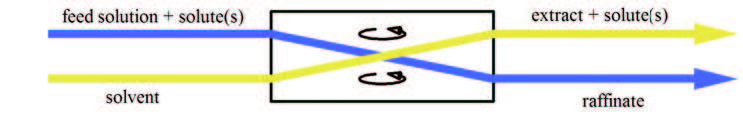
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or
metal complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. Man ...
es, based on their relative
solubilities in two different
immiscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more
species
In biology, a species is the basic unit of Taxonomy (biology), classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of ...
from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the
raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from
separatory funnels
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two immisci ...
to
countercurrent distribution Countercurrent distribution (CCD, also spelled "counter current" distribution) is an analytical chemistry technique which was developed by Lyman C. Craig in the 1940s. Countercurrent distribution is a separation process that is founded on the princi ...
equipment called as
mixer settlers. This type of process is commonly performed after a chemical reaction as part of the
work-up
In chemistry, work-up refers to the series of manipulations required to isolate and Chemical purity, purify the Product (chemistry), product(s) of a chemical reaction.
Typically, these manipulations may include:
* quenching a reaction to deactiva ...
, often including an acidic work-up.
The term ''partitioning'' is commonly used to refer to the underlying chemical and physical processes involved in ''liquid–liquid extraction'', but on another reading may be fully synonymous with it. The term ''solvent extraction'' can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.
From a
hydrometallurgical perspective, solvent extraction is exclusively used in separation and purification of uranium and plutonium, zirconium and hafnium, separation of cobalt and nickel, separation and purification of rare earth elements etc., its greatest advantage being its ability to selectively separate out even very similar metals. One obtains high-purity single metal streams on 'stripping' out the metal value from the 'loaded' organic wherein one can precipitate or deposit the metal value. Stripping is the opposite of extraction: Transfer of mass from organic to aqueous phase.
LLE is also widely used in the production of fine
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s, the processing of
perfume
Perfume (, ; french: parfum) is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. ...
s, the production of
vegetable oils
Vegetable oils, or vegetable fats, are oils extracted from seeds or from other parts of fruits. Like animal fats, vegetable fats are ''mixtures'' of triglycerides. Soybean oil, grape seed oil, and cocoa butter are examples of seed oils, o ...
and
biodiesel
Biodiesel is a form of diesel fuel derived from plants or animals and consisting of long-chain fatty acid esters. It is typically made by chemically reacting lipids such as animal fat ( tallow), soybean oil, or some other vegetable oil ...
, and other industries. It is among the most common initial separation techniques, though some difficulties result in extracting out closely related functional groups.
Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with
molten salt
Molten salt is salt which is solid at standard temperature and pressure but enters the liquid phase due to elevated temperature. Regular table salt has a melting point of 801 °C (1474°F) and a heat of fusion of 520 J/g.Proc. Roy. Soc. Bibli ...
s, metals can be extracted from one phase to the other. This is related to a
mercury electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
where a metal can be reduced, the metal will often then dissolve in the mercury to form an
amalgam that modifies its electrochemistry greatly. For example, it is possible for
sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s to be reduced at a mercury
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction i ...
to form
sodium amalgam
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium a ...
, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A
detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
or fine
solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structura ...
can be used to stabilize an
emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althou ...
, or
third phase.
Measures of effectiveness
Distribution ratio
In solvent extraction, a distribution ratio is often quoted as a measure of how well-extracted a species is. The distribution ratio (''Kd'') is equal to the
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
of a
solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solve ...
in the organic phase divided by its concentration in the
aqueous phase. Depending on the system, the distribution ratio can be a function of temperature, the concentration of chemical species in the system, and a large number of other parameters. Note that ''D'' is related to the Δ''G'' of the extraction process.
Sometimes, the distribution ratio is referred to as the
partition coefficient
In the physical sciences, a partition coefficient (''P'') or distribution coefficient (''D'') is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solub ...
, which is often expressed as the
logarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 of ...
. Note that a distribution ratio for
uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
and
neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being ...
between two inorganic solids (
zirconolite
Zirconolite is a mineral, calcium zirconium titanate; formula CaZrTi2O7. Some examples of the mineral may also contain thorium, uranium, cerium, niobium and iron; the presence of thorium or uranium would make the mineral radioactive
Radioac ...
and
perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure
In crystallography, crystal ...
) has been reported.
In solvent extraction, two immiscible liquids are shaken together. The more
polar solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. In this experiment, the nonpolar halogens preferentially dissolve in the non-polar mineral oil.
Although the distribution ratio and partition coefficient are often used synonymously, they are not necessarily so. Solutes may exist in more than one form in any particular phase, which would mean that the partition coefficient (Kd) and distribution ratio (D) will have different values. This is an important distinction to make as whilst the partition coefficient has a fixed value for the partitioning of a solute between two phases, the distribution ratio changes with differing conditions in the solvent.
After performing liquid–liquid extraction, a quantitative measure must be taken to determine the ratio of the solution's total concentration in each phase of the extraction. This quantitative measure is known as the distribution ratio or distribution coefficient.
Separation factors
The separation factor is one distribution ratio divided by another; it is a measure of the ability of the system to separate two solutes. For instance, if the distribution ratio for
nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
(D
Ni) is 10 and the distribution ratio for
silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
(D
Ag) is 100, then the silver/nickel separation factor (SF
Ag/Ni) is equal to D
Ag/D
Ni = SF
Ag/Ni = 10.
Decontamination factor
This is used to express the ability of a process to remove a
contaminant
Contamination is the presence of a constituent, impurity, or some other undesirable element that spoils, corrupts, infects, makes unfit, or makes inferior a material, physical body, natural environment, workplace, etc.
Types of contamination
Wi ...
from a product. For instance, if a process is fed with a mixture of 1:9
cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
to
indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 par ...
, and the product is a 1:99 mixture of cadmium and indium, then the decontamination factor (for the removal of cadmium) of the process is 0.11 / 0.01 = 11.
Slopes of graphs
The easy way to work out the extraction mechanism is to draw graphs and measure the slopes. If for an extraction system the ''D'' value is proportional to the square of the concentration of a reagent (''Z'') then the slope of the graph of log
10(''D'') against log
10(
''Z''
) will be two.
Measures of success
Success of liquid–liquid extraction is measured through separation factors and decontamination factors. The best way to understand the success of an extraction column is through the liquid–liquid equilibrium (LLE) data set. The data set can then be converted into a curve to determine the steady state partitioning behavior of the solute between the two phases. The y-axis is the concentration of solute in the extract (solvent) phase, and the x-axis is the concentration of the solute in the raffinate phase. From here, one can determine steps for optimization of the process.
Techniques
Batchwise single stage extractions
This is commonly used on the small scale in chemical labs. It is normal to use a
separating funnel
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two immisci ...
. Processes include DLLME and direct organic extraction.
After equilibration, the extract phase containing the desired solute is separated out for further processing.
Dispersive liquid–liquid microextraction (DLLME)
A process used to extract small amounts of organic compounds from water samples.
This process is done by injecting small amounts of an appropriate extraction solvent (C
2Cl
4) and a disperser solvent (acetone) into the aqueous solution. The resulting solution is then
centrifuge
A centrifuge is a device that uses centrifugal force to separate various components of a fluid. This is achieved by spinning the fluid at high speed within a container, thereby separating fluids of different densities (e.g. cream from milk) or ...
d to separate the organic and aqueous layers. This process is useful in extraction organic compounds such as organochloride and organophsophorus pesticides, as well as substituted benzene compounds from water samples.
Direct organic extraction
By mixing partially organic soluble samples in organic solvent (toluene, benzene, xylene), the organic soluble compounds will dissolve into the solvent and can be separated using a
separatory funnel
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two immiscib ...
. This process is valuable in the extraction of proteins and specifically phosphoprotein and phosphopeptide phosphatases.
Another example of this application is extracting
anisole
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is m ...
from a
mixture of
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
and 5%
acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
using
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again b ...
, then the anisole will enter the organic phase. The two phases would then be separated. The acetic acid can then be scrubbed (removed) from the organic phase by shaking the organic extract with
sodium bicarbonate. The acetic acid reacts with the sodium bicarbonate to form
sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
,
carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, and water.
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
can also be extracted from coffee beans and tea leaves using a direct organic extraction. The beans or leaves can be soaked in ethyl acetate which favorably dissolves the caffeine, leaving a majority of the coffee or tea flavor remaining in the initial sample.
Multistage countercurrent continuous processes
These are commonly used in
industry for the processing of
metals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
such as the
lanthanides; because the separation factors between the lanthanides are so small many extraction stages are needed.
In the multistage processes, the aqueous
raffinate from one extraction unit is fed to the next unit as the aqueous feed, while the organic phase is moved in the opposite direction. Hence, in this way, even if the separation between two metals in each stage is small, the overall system can have a higher decontamination factor.
Multistage
countercurrent arrays have been used for the separation of lanthanides. For the design of a good process, the distribution ratio should be not too high (>100) or too low (<0.1) in the extraction portion of the process. It is often the case that the process will have a section for scrubbing unwanted metals from the organic phase, and finally a
stripping section to obtain the metal back from the organic phase.
Mixer–settlers
Battery of
mixer-settlers counter currently interconnected. Each mixer-settler unit provides a single stage of extraction. A mixer settler consists of a first stage that mixes the phases together followed by a quiescent settling stage that allows the phases to separate by gravity.

Continuous separation of oil& water mixture
A novel settling device
Sudhin BioSettler canseparate an oil-water emulsion continuously at a much faster rate than simple gravity settlers. In this photo, an oil-water emulsion, stirred by an impeller in an external reservoir and pumped continuously into the two bottom side ports of BioSettler, is separated very quickly into a clear organic (mineral oil) layer exiting via the top of BioSettler and an aqueous (coloured with a red food dye) layer being pumped out continuously from the bottom of BioSettler.
In the multistage countercurrent process, multiple mixer settlers are installed with mixing and settling chambers located at alternating ends for each stage (since the outlet of the settling sections feed the inlets of the adjacent stage's mixing sections). Mixer-settlers are used when a process requires longer residence times and when the solutions are easily separated by gravity. They require a large facility footprint, but do not require much headspace, and need limited remote maintenance capability for occasional replacement of mixing motors. (Colven, 1956; Davidson, 1957)
[Liquid–Liquid Extraction Equipment](_blank)
Jack D. Law and Terry A. Todd, Idaho National Laboratory.

Centrifugal extractors
Centrifugal extractors mix and separate in one unit. Two liquids will be intensively mixed between the spinning rotor and the stationary housing at speeds up to 6000 RPM. This develops great surfaces for an ideal mass transfer from the aqueous phase into the organic phase. At 200–2000 g, both phases will be separated again. Centrifugal extractors minimize the solvent in the process, optimize the product load in the solvent and extract the aqueous phase completely. Counter current and cross current extractions are easily established.
Extraction without chemical change
Some solutes such as
noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low che ...
es can be extracted from one phase to another without the need for a chemical reaction (see
absorption). This is the simplest type of solvent extraction. When a solvent is extracted, two immiscible liquids are shaken together. The more polar solutes dissolve preferentially in the more polar solvent, and the less polar solutes in the less polar solvent. Some solutes that do not at first sight appear to undergo a reaction during the extraction process do not have distribution ratio that is independent of concentration. A classic example is the extraction of
carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
(HA) into nonpolar media such as
benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
. Here, it is often the case that the carboxylic acid will form a dimer in the organic layer so the distribution ratio will change as a function of the acid concentration (measured in either phase).
For this case, the extraction constant ''k'' is described by ''k'' =
HAorganic2/
HAaqueous
Solvation mechanism
Using solvent extraction it is possible to extract
uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
,
plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhib ...
,
thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
and many rare earth elements from acid solutions in a selective way by using the right choice of organic extracting solvent and diluent. One solvent used for this purpose is the
organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered ...
tributyl phosphate (TBP). The
PUREX
PUREX (plutonium uranium reduction extraction) is a chemistry, chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the ''de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and p ...
process that is commonly used in
nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, ...
uses a mixture of tri-n-butyl phosphate and an
inert
Inert may refer to:
* Chemically inert, not chemically reactive
** Inert gas
** Noble gas, historically called inert gas
* Inert knowledge, information which one can express but not use
* Inert waste, waste which is neither chemically nor biol ...
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
(
kerosene
Kerosene, paraffin, or lamp oil is a combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in aviation as well as households. Its name derives from el, κηρός (''keros'') meaning " wax", and was reg ...
), the uranium(VI) are extracted from strong
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
and are back-extracted (stripped) using weak nitric acid. An organic soluble uranium
complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
O2(TBP)2(NO3)2is formed, then the organic layer bearing the uranium is brought into contact with a
dilute
Dilution may refer to:
* Reducing the concentration of a chemical
* Serial dilution, a common way of going about this reduction of concentration
* Homeopathic dilution
* Dilution (equation), an equation to calculate the rate a gas dilutes
* Trad ...
nitric acid solution; the equilibrium is shifted away from the organic soluble uranium complex and towards the free TBP and
uranyl nitrate in dilute nitric acid. The plutonium(IV) forms a similar complex to the uranium(VI), but it is possible to strip the plutonium in more than one way; a
reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth m ...
that converts the plutonium to the trivalent
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
can be added. This oxidation state does not form a stable complex with TBP and
nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble ...
unless the nitrate concentration is very high (circa 10 mol/L nitrate is required in the aqueous phase). Another method is to simply use dilute nitric acid as a stripping agent for the plutonium. This PUREX chemistry is a classic example of a
solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the ...
extraction Extraction may refer to:
Science and technology
Biology and medicine
* Comedo extraction, a method of acne treatment
* Dental extraction, the surgical removal of a tooth from the mouth
Computing and information science
* Data extraction, the pro ...
.
Here in this case D
U = k TBP
2 NO32
Ion exchange mechanism
Another extraction mechanism is known as the
ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
mechanism. Here, when an ion is transferred from the aqueous phase to the organic phase, another
ion is transferred in the other direction to maintain the charge balance. This additional ion is often a
hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
; for ion exchange mechanisms, the distribution ratio is often a function of
pH. An example of an ion exchange extraction would be the extraction of
americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
by a combination of
terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemis ...
and a
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
in ''tert''-
butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
. In this case
''D''
Am = ''k''
terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemis ...
1carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
3 H+−3
Another example is the extraction of
zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
,
cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
, or
lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate ...
by a di
alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloal ...
phosphinic acid (R
2PO
2H) into a nonpolar
diluent
A diluent (also referred to as a filler, dilutant or thinner) is a diluting agent. Certain fluids are too viscous to be pumped easily or too dense to flow from one particular point to the other. This can be problematic, because it might not be e ...
such as an
alkane
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms tha ...
. A non-
polar diluent favours the formation of uncharged non-polar
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
complexes.
Some extraction systems are able to extract metals by both the solvation and ion exchange mechanisms; an example of such a system is the americium (and
lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and y ...
) extraction from
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
by a combination of 6,6'-''bis''-(5,6-di
pentyl
Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane.
In older literature, the common non-systematic name amyl was often used for the pentyl group. Conversely, the na ...
-1,2,4-triazin-3-yl)-
2,2'-bipyridine
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
and 2-bromo
hexanoic acid in ''tert''-
butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
. At both high- and low-nitric acid concentrations, the metal distribution ratio is higher than it is for an intermediate nitric acid concentration.
Ion pair extraction
It is possible by careful choice of counterion to extract a metal. For instance, if the
nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble ...
concentration is high, it is possible to extract
americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
as an
anionic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
nitrate complex if the mixture contains a
lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves li ...
quaternary ammonium salt
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cat ...
.
An example that is more likely to be encountered by the '' 'average' '' chemist is the use of a
phase transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic ...
. This is a charged species that transfers another
ion to the organic phase. The ion reacts and then forms another ion, which is then transferred back to the aqueous phase.
For instance, the 31.1
kJ mol−1 is required to transfer an
acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
anion into nitrobenzene, while the energy required to transfer a chloride anion from an aqueous phase to nitrobenzene is 43.8 kJ mol
−1. Hence, if the aqueous phase in a reaction is a solution of
sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
while the organic phase is a nitrobenzene solution of
benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Preparation
Benzyl chloride is prepared indu ...
, then, when a phase transfer catalyst, the acetate anions can be transferred from the aqueous layer where they react with the
benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a subst ...
chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
to form benzyl acetate and a chloride anion. The chloride anion is then transferred to the aqueous phase. The transfer energies of the anions contribute to that given out by the reaction.
A 43.8 to 31.1 kJ mol
−1 = 12.7 kJ mol
−1 of additional energy is given out by the reaction when compared with energy if the reaction had been done in
nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor ...
using one
equivalent weight
In chemistry, equivalent weight (also known as gram equivalent) is the mass of one equivalent, that is the mass of a given substance which will combine with or displace a fixed quantity of another substance. The equivalent weight of an element i ...
of a
tetraalkylammonium acetate.
Types of aqueous two-phase extractions
Polymer–polymer systems. In a Polymer–polymer system, both phases are generated by a dissolved polymer. The heavy phase will generally be a
polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with ...
, and the light phase is generally
Polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular w ...
(PEG). Traditionally, the polysaccharide used is
dextran
Dextran is a complex branched glucan (polysaccharide derived from the condensation of glucose), originally derived from wine. IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 � ...
. However, dextran is relatively expensive, and research has been exploring using less expensive polysaccharides to generate the heavy phase. If the target compound being separated is a protein or enzyme, it is possible to incorporate a ligand to the target into one of the polymer phases. This improves the target's affinity to that phase, and improves its ability to partition from one phase into the other. This, as well as the absence of solvents or other denaturing agents, makes polymer–polymer extractions an attractive option for purifying proteins. The two phases of a polymer–polymer system often have very similar densities, and very low surface tension between them. Because of this, demixing a polymer–polymer system is often much more difficult than demixing a solvent extraction. Methods to improve the demixing include
centrifugation
Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate ...
, and application of an
electric field.
Polymer–salt systems. Aqueous two-phase systems can also be generated by generating the heavy phase with a concentrated salt solution. The polymer phase used is generally still PEG. Generally, a
kosmotropic salt, such as Na
3PO
4 is used, however PEG–NaCl systems have been documented when the salt concentration is high enough. Since polymer–salt systems demix readily they are easier to use. However, at high salt concentrations, proteins generally either denature, or precipitate from solution. Thus, polymer–salt systems are not as useful for purifying proteins.
Ionic liquids systems.
Ionic liquids are ionic compounds with low melting points. While they are not technically aqueous, recent research has experimented with using them in an extraction that does not use organic solvents.
DNA purification
The ability to purify DNA from a sample is important for many modern biotechnology processes. However, samples often contain nucleases that degrade the target DNA before it can be purified. It has been shown that DNA fragments will partition into the light phase of a polymer–salt separation system. If ligands known to bind and deactivate nucleases are incorporated into the polymer phase, the nucleases will then partition into the heavy phase and be deactivated. Thus, this polymer–salt system is a useful tool for purifying DNA from a sample while simultaneously protecting it from nucleases.
Food industry
The PEG–NaCl system has been shown to be effective at partitioning small molecules, such as peptides and nucleic acids. These compounds are often flavorants or odorants. The system could then be used by the food industry to isolate or eliminate particular flavors.
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
extraction used to be done using liquid–liquid extraction, specifically direct and indirect liquid–liquid extraction (Swiss Water Method), but has since moved towards super-critical CO
2 as it is cheaper and can be done on a commercial scale.
Analytical chemistry
Often there are chemical species present or necessary at one stage of sample processing that will interfere with the analysis. For example, some air monitoring is performed by drawing air through a small glass tube filled with sorbent particles that have been coated with a chemical to stabilize or derivatize the analyte of interest. The coating may be of such a concentration or characteristics that it would damage the instrumentation or interfere with the analysis. If the sample can be extracted from the sorbent using a nonpolar solvent (such as toluene or carbon disulfide), and the coating is polar (such as HBr or phosphoric acid) the dissolved coating will partition into the aqueous phase. Clearly the reverse is true as well, using polar extraction solvent and a nonpolar solvent to partition a nonpolar interferent. A small aliquot of the organic phase (or in the latter case, polar phase) can then be injected into the instrument for analysis.
Purification of amines
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent su ...
s (analogously to ammonia) have a lone pair of electrons on the nitrogen atom that can form a relatively weak bond to a hydrogen atom. It is therefore the case that under acidic conditions amines are typically protonated, carrying a positive charge and under basic conditions they are typically deprotonated and neutral. Amines of sufficiently low molecular weight are rather polar and can form hydrogen bonds with water and therefore will readily dissolve in aqueous solutions. Deprotonated amines on the other hand, are neutral and have ''greasy'', nonpolar organic substituents, and therefore have a higher affinity for nonpolar inorganic solvents. As such purification steps can be carried out where an aqueous solution of an amine is neutralized with a base such as sodium hydroxide, then shaken in a
separatory funnel
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two immiscib ...
with a nonpolar solvent that is immiscible with water. The organic phase is then drained off. Subsequent processing can recover the amine by techniques such as recrystallization, evaporation or distillation; subsequent extraction back to a polar phase can be performed by adding HCl and shaking again in a separatory funnel (at which point the ammonium ion could be recovered by adding an insoluble counterion), or in either phase, reactions could be performed as part of a chemical synthesis.
Temperature swing solvent extraction
Temperature swing solvent extraction is an experimental technique for the desalination of drinking water. It has been used to remove up to 98.4% of the salt content in water, and is able to process hypersaline brines that cannot be desalinated using reverse osmosis.
Kinetics of extraction
It is important to investigate the rate at which the solute is transferred between the two phases, in some cases by an alteration of the contact time it is possible to alter the selectivity of the extraction. For instance, the extraction of
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
or
nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
can be very slow because the rate of ligand exchange at these metal centers is much lower than the rates for
iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
or
silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
complexes.
Aqueous complexing agents
If a complexing agent is present in the aqueous phase then it can lower the distribution ratio. For instance, in the case of iodine being distributed between water and an inert organic solvent such as
carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
then the presence of
iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine def ...
in the aqueous phase can alter the extraction chemistry.
Instead of
being a constant it becomes
= ''k''
I2.Organic/
2.Aqueous I−.Aqueous
This is because the
iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
reacts with the
iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine def ...
to form
I3−. The I
3− anion is an example of a
polyhalide anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
that is quite common.
Industrial process design
In a typical scenario, an industrial process will use an extraction step in which solutes are transferred from the aqueous phase to the organic phase; this is often followed by a scrubbing stage in which unwanted solutes are removed from the organic phase, then a stripping stage in which the wanted solutes are removed from the organic phase. The organic phase may then be treated to make it ready for use again.
After use, the organic phase may be subjected to a cleaning step to remove any degradation products; for instance, in PUREX plants, the used organic phase is washed with
sodium carbonate solution to remove any dibutyl hydrogen phosphate or butyl dihydrogen phosphate that might be present.
Liquid-liquid equilibrium calculations
In order to calculate the phase equilibrium, it is necessary to use a thermodynamic model such as NRTL, UNIQUAC, etc. The corresponding parameters of these models can be obtained from literature (e.g. Dechema Chemistry Data Series,
Dortmund Data Bank
The Dortmund Data Bank (short DDB) is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimizat ...
, etc.) or by a correlation process of experimental data.
Equipment
While solvent extraction is often done on a small scale by synthetic lab chemists using a
separatory funnel
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two immiscib ...
,
Craig apparatus or membrane-based techniques, it is normally done on the industrial scale using machines that bring the two liquid phases into contact with each other. Such machines include
centrifugal contactor
A centrifugal extractor—also known as a centrifugal contactor or annular centrifugal contactor—uses the rotation of the rotor inside a centrifuge to mix two immiscible liquids outside the rotor and to separate the liquids in the field of gra ...
s,
Thin Layer Extraction,
spray columns,
pulsed columns
Pulsed columns are a type of liquid-liquid extraction equipment; examples of this class of extraction equipment is used at the BNFL plant THORP
''Thorp'' is a Middle English word for a hamlet or small village.
Etymology
The name can either ...
, and
mixer-settlers.
Extraction of metals
The extraction methods for a range of metals include:
Cobalt
The extraction of cobalt from
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
using
Alamine 336 (tri-octyl/decyl amine) in ''
meta
Meta (from the Greek μετά, '' meta'', meaning "after" or "beyond") is a prefix meaning "more comprehensive" or "transcending".
In modern nomenclature, ''meta''- can also serve as a prefix meaning self-referential, as a field of study or ende ...
''-
xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are sub ...
. Cobalt can be extracted also using
Cyanex 272 .
Copper
Copper can be extracted using hydroxy
oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substitu ...
s as extractants, a recent paper describes an extractant that has a good selectivity for copper over
cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
and
nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
.
Neodymium
The rare earth element Neodymium is extracted by di(2-ethyl-hexyl)phosphoric acid into
hexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
by an ion exchange mechanism.
Nickel
Nickel can be extracted using di(2-ethyl-hexyl)phosphoric acid and
tributyl phosphate in a hydrocarbon diluent (Shellsol).
Palladium and platinum
Dialkyl sulfides, tributyl phosphate and alkyl amines have been used for extracting palladium and platinum.
Polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic characte ...
is produced in reactors from natural
209Bi, bombarded with
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s, creating
210Bi, which then decays to
210Po via beta-minus decay. The final purification is done pyrochemically followed by liquid-liquid extraction vs sodium hydroxide at 500 deg C.
Zinc and cadmium
Zinc and cadmium are both extracted by an ion exchange process, the
''N,N,N′,N′''-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) acts as a masking agent for the zinc and an extractant for the cadmium. In the modified Zincex process, zinc is separated from most divalent ions by solvent extraction. D2EHPA (Di (2) ethyl hexyl phosphoric acid) is used for this. A zinc ion replaces the proton from two D2EHPA molecules. To strip the zinc from the D2EHPA, sulfuric acid is used, at a concentration of above 170g/L (typically 240-265g/L).
Lithium
Lithium extraction is more popular due to the high demand of
lithium-ion batteries
A lithium-ion or Li-ion battery is a type of rechargeable battery which uses the reversible reduction of lithium ions to store energy. It is the predominant battery type used in portable consumer electronics and electric vehicles. It also see ...
.
TBP (Tri-butyl phosphate) and FeCl3 are mostly used to extract lithium from brine (with high Li/Mg ratio). Alternatively, Cyanex 272 was also used to extract lithium. The mechanism of lithium extraction was found differently from other metals, such as cobalt, due to the weak coordinating bonding between lithium ions and extractants.
See also
*
Fragrance extraction
*
Dortmund Data Bank
The Dortmund Data Bank (short DDB) is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimizat ...
*
Non-random two-liquid model - (NRTL model) LL Phase Equilibrium Calculation
*
UNIQUAC - LL Phase Equilibrium Calculation
References
Further reading
*B.L. Karger, 2014, "Separation and Purification: Single-stage versus multistage processes" and "Separation and Purification: Separations Based on Equilibrium", Encyclopædia Britannica, se
an
accessed 12 May 2014.
*Gunt Hamburg, 2014, "Thermal Process Engineering: liquid–liquid extraction and solid-liquid extraction", se
accessed 12 May 2014.
*G.W. Stevens, T.C., Lo, & M. H. I. Baird, 2007, "Extraction, liquid–liquid", in Kirk-Othmer Encyclopedia of Chemical Technology, , accessed 12 May 2014.
*Colin Poole & Michael Cooke, 2000, "Extraction", in Encyclopedia of Separation Science, 10 Vols., , se
accessed 12 May 2014.
*Sikdar, Cole, et al. Aqueous Two-Phase Extractions in Bioseparations: An Assessment. Biotechnology 9:254. 1991
*Szlag, Giuliano. A Low-Cost Aqueous Two Phase System for Enzyme Extraction. Biotechnology Techniques 2:4:277. 1988
*Dreyer, Kragl. Ionic Liquids for Aqueous Two-Phase Extraction and Stabilization of Enzymes. Biotechnology and Bioengineering. 99:6:1416. 2008
*Boland. Aqueous Two-Phase Systems: Methods and Protocols. Pg 259-269
*https://web.archive.org/web/20100702074135/http://ull.chemistry.uakron.edu/chemsep/extraction/
Topological Analysis of the Gibbs Energy Function (Liquid-Liquid Equilibrium Correlation Data). Including a Thermodynamic Review and a Graphical User Interface (GUI) for Surfaces/Tie-lines/Hessian matrix analysis- University of Alicante (Reyes-Labarta et al. 2015-18)
{{DEFAULTSORT:Liquid-liquid extraction
Extraction (chemistry)
Laboratory techniques
Flavor technology
Articles containing video clips
 Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or  Continuous separation of oil& water mixture
A novel settling device
Continuous separation of oil& water mixture
A novel settling device