Lewis Dot Structures on:
[Wikipedia]
[Google]
[Amazon]
 Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between
 Chemical structures may be written in more compact forms, particularly when showing
Chemical structures may be written in more compact forms, particularly when showing
Lewis Dot Diagrams of Selected Elements
{{Chemical bonding theory 1916 introductions Chemical formulas Chemical bonding
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s of a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
, as well as the lone pairs
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s that may exist in the molecule. A Lewis structure can be drawn for any covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
ly bonded molecule, as well as coordination compounds
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
. The Lewis structure was named after Gilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
, who introduced it in his 1916 article ''The Atom and the Molecule.'' Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pair
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s in a chemical bond.
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Although main group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 compos ...
of (8) electrons, hydrogen (H) can only form bonds which share just two electrons.
Construction and electron counting
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom. Non-valence electrons are not represented in Lewis structures. Once the total number of available electrons has been determined, electrons must be placed into the structure according to these steps: # The atoms are first connected by single bonds. # If ''t'' is the total number of electrons and ''n'' the number of single bonds, ''t-2n'' electrons remain to be placed. These should be placed as lone pairs: one pair of dots for each pair of electrons available. Lone pairs should initially be placed on outer atoms (other than hydrogen) until each outer atom has ''eight'' electrons in bonding pairs and lone pairs; extra lone pairs may then be placed on the central atom. When in doubt, lone pairs should be placed on more electronegative atoms first. # Once all lone pairs are placed, atoms (especially the central atoms) may not have an octet of electrons. In this case, the atoms must form a double bond; a lone pair of electrons is moved to form a second bond between the two atoms. As the bonding pair is shared between the two atoms, the atom that originally had the lone pair still has an octet; the other atom now has two more electrons in its valence shell. Lewis structures for polyatomic ions may be drawn by the same method. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside the brackets. A simpler method has been proposed for constructing Lewis structures, eliminating the need for electron counting: the atoms are drawn showing the valence electrons; bonds are then formed by pairing up valence electrons of the atoms involved in the bond-making process, and anions and cations are formed by adding or removing electrons to/from the appropriate atoms. A trick is to count up valence electrons, then count up the number of electrons needed to complete the octet rule (or with hydrogen just 2 electrons), then take the difference of these two numbers. The answer is the number of electrons that make up the bonds. The rest of the electrons just go to fill all the other atoms' octets. Another simple and general procedure to write Lewis structures and resonance forms has been proposed.Formal charge
In terms of Lewis structures,formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
is used in the description, comparison, and assessment of likely topological and resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
structuresMiessler, G. L. and Tarr, D. A., ''Inorganic Chemistry'' (2nd ed., Prentice Hall 1998) , pp. 49–53 – Explanation of formal charge usage. by determining the apparent electronic charge of each atom within, based upon its electron dot structure, assuming exclusive covalency or non-polar bonding. It has uses in determining possible electron re-configuration when referring to reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
s, and often results in the same sign as the partial charge of the atom, with exceptions. In general, the formal charge of an atom can be calculated using the following formula, assuming non-standard definitions for the markup used:
:
where:
* is the formal charge.
* represents the number of valence electrons in a free atom of the element.
* represents the number of unshared electrons on the atom.
* represents the total number of electrons in bonds the atom has with another.
The formal charge of an atom is computed as the difference between the number of valence electrons that a neutral atom would have and the number of electrons that belong to it in the Lewis structure. Electrons in covalent bonds are split equally between the atoms involved in the bond. The total of the formal charges on an ion should be equal to the charge on the ion, and the total of the formal charges on a neutral molecule should be equal to zero.
Resonance
For some molecules and ions, it is difficult to determine which lone pairs should be moved to form double or triple bonds, and two or more different ''resonance'' structures may be written for the same molecule or ion. In such cases it is usual to write all of them with two-way arrows in between (see Example below). This is sometimes the case when multiple atoms of the same type surround the central atom, and is especially common for polyatomic ions. When this situation occurs, the molecule's Lewis structure is said to be aresonance structure
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance stru ...
, and the molecule exists as a resonance hybrid. Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to some combination of these states.
The nitrate ion (NO3−), for instance, must form a double bond between nitrogen and one of the oxygens to satisfy the octet rule for nitrogen. However, because the molecule is symmetrical, it does not matter ''which'' of the oxygens forms the double bond. In this case, there are three possible resonance structures. Expressing resonance when drawing Lewis structures may be done either by drawing each of the possible resonance forms and placing double-headed arrows between them or by using dashed lines to represent the partial bonds (although the latter is a good representation of the resonance hybrid which is not, formally speaking, a Lewis structure).
When comparing resonance structures for the same molecule, usually those with the fewest formal charges contribute more to the overall resonance hybrid. When formal charges are necessary, resonance structures that have negative charges on the more electronegative elements and positive charges on the less electronegative elements are favored.
Single bonds can also be moved in the same way to create resonance structures for hypervalent molecules
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus p ...
such as sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attached ...
, which is the correct description according to quantum chemical calculations instead of the common expanded octet model.
The resonance structure should not be interpreted to indicate that the molecule switches between forms, but that the molecule acts as the average of multiple forms.
Example
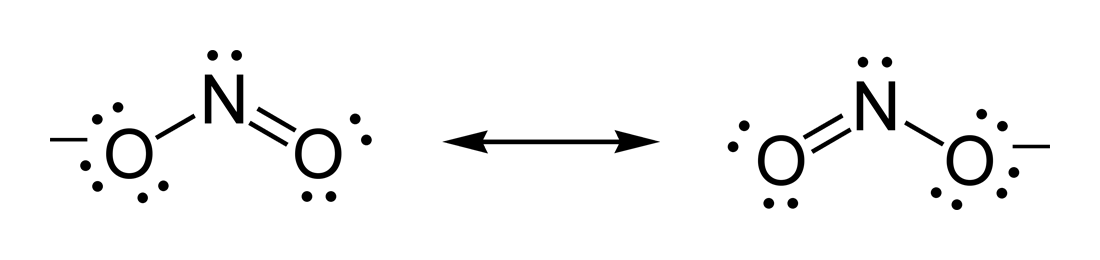
The formula of thenitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
ion is .
# Nitrogen is the least electronegative atom of the two, so it is the central atom by multiple criteria.
# Count valence electrons. Nitrogen has 5 valence electrons; each oxygen has 6, for a total of (6 × 2) + 5 = 17. The ion has a charge of −1, which indicates an extra electron, so the total number of electrons is 18.
# Connect the atoms by single bonds. Each oxygen must be bonded to the nitrogen, which uses four electrons—two in each bond.
# Place lone pairs. The 14 remaining electrons should initially be placed as 7 lone pairs. Each oxygen may take a maximum of 3 lone pairs, giving each oxygen 8 electrons including the bonding pair. The seventh lone pair must be placed on the nitrogen atom.
# Satisfy the octet rule. Both oxygen atoms currently have 8 electrons assigned to them. The nitrogen atom has only 6 electrons assigned to it. One of the lone pairs on an oxygen atom must form a double bond, but either atom will work equally well. Therefore, there is a resonance structure.
# Tie up loose ends. Two Lewis structures must be drawn: Each structure has one of the two oxygen atoms double-bonded to the nitrogen atom. The second oxygen atom in each structure will be single-bonded to the nitrogen atom. Place brackets around each structure, and add the charge (−) to the upper right outside the brackets. Draw a double-headed arrow between the two resonance forms.

Alternative formations
 Chemical structures may be written in more compact forms, particularly when showing
Chemical structures may be written in more compact forms, particularly when showing organic molecules
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
. In condensed structural formulas, many or even all of the covalent bonds may be left out, with subscripts indicating the number of identical groups attached to a particular atom.
Another shorthand structural diagram is the skeletal formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A ...
(also known as a bond-line formula or carbon skeleton diagram). In a skeletal formula, carbon atoms are not signified by the symbol C but by the vertices of the lines. Hydrogen atoms bonded to carbon are not shown—they can be inferred by counting the number of bonds to a particular carbon atom—each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to hydrogen atoms.
Other diagrams may be more complex than Lewis structures, showing bonds in 3D using various forms such as space-filling diagrams.
Usage and limitations
Despite their simplicity and development in the early twentieth century, when understanding of chemical bonding was still rudimentary, Lewis structures capture many of the key features of the electronic structure of a range of molecular systems, including those of relevance to chemical reactivity. Thus, they continue to enjoy widespread use by chemists and chemistry educators. This is especially true in the field oforganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, where the traditional valence-bond model of bonding still dominates, and mechanisms are often understood in terms of curve-arrow notation superimposed upon skeletal formulae, which are shorthand versions of Lewis structures. Due to the greater variety of bonding schemes encountered in inorganic and organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, many of the molecules encountered require the use of fully delocalized molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
to adequately describe their bonding, making Lewis structures comparatively less important (although they are still common).
It is important to note that there are simple and archetypal molecular systems for which a Lewis description, at least in unmodified form, is misleading or inaccurate. Notably, the naive drawing of Lewis structures for molecules known experimentally to contain unpaired electrons (e.g., O2, NO, and ClO2) leads to incorrect inferences of bond orders, bond lengths, and/or magnetic properties. A simple Lewis model also does not account for the phenomenon of aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturate ...
. For instance, Lewis structures do not offer an explanation for why cyclic C6H6 (benzene) experiences special stabilization beyond normal delocalization effects, while C4H4 (cyclobutadiene) actually experiences a special ''destabilization''. Molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
provides the most straightforward explanation for these phenomena.
See also
* Valence shell electron pair repulsion theory * Molecular geometry *Structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bondi ...
* Natural bond orbital
References
External links
*Lewis Dot Diagrams of Selected Elements
{{Chemical bonding theory 1916 introductions Chemical formulas Chemical bonding