Jirō Tsuji on:
[Wikipedia]
[Google]
[Amazon]
Jiro Tsuji (May 11, 1927 – April 1, 2022) was a Japanese chemist, notable for his discovery of organometallic reactions, including the
 His pioneering work resulting in the development of the
His pioneering work resulting in the development of the  Later research hinged on the discovery that allyl acetoacetates decarboxylate and regioselectively ''alpha''-allylate in the presence of catalytic Pd(OAc)2 and PPh3 ''via'' the intermediacy of an allylpalladium enolate - a catalytic
Later research hinged on the discovery that allyl acetoacetates decarboxylate and regioselectively ''alpha''-allylate in the presence of catalytic Pd(OAc)2 and PPh3 ''via'' the intermediacy of an allylpalladium enolate - a catalytic
Tsuji–Trost reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coor ...
, the Tsuji–Wilkinson decarbonylation reaction, and the Tsuji–Wacker reaction.
Early life and education
Tsuji was born in Japan in 1927. After attendingKyoto University
, or , is a National university, national research university in Kyoto, Japan. Founded in 1897, it is one of the former Imperial Universities and the second oldest university in Japan.
The university has ten undergraduate faculties, eighteen gra ...
, Tsuji began his doctoral research at Columbia University
Columbia University in the City of New York, commonly referred to as Columbia University, is a Private university, private Ivy League research university in New York City. Established in 1754 as King's College on the grounds of Trinity Churc ...
under Gilbert Stork
Gilbert Stork (December 31, 1921 – October 21, 2017) was a Belgian-American organic chemist. For a quarter of a century he was the Eugene Higgins Professor of Chemistry Emeritus at Columbia University. He is known for making significant contri ...
studying natural product synthesis, and making contributions to research on the dissolving metal reduction of enones.
Independent career
His independent career began at Toyo Rayon (nowToray Industries
is a multinational corporation headquartered in Japan that specializes in industrial products centered on technologies in organic synthetic chemistry, polymer chemistry, and biochemistry.
Its founding business areas were fibers and textiles, ...
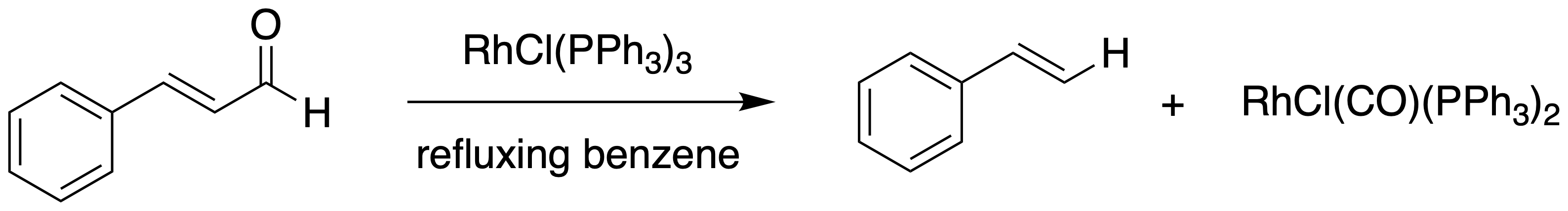
) studying the stoichiometric, and later catalytic carbonylation of PdII-alkene complexes with carbon monoxide. His preliminary results showed that acyl halides and aldehydes could be decarbonylated by Pd0 at high temperatures (200 °C) yielding alkenes; further investigation revealed that stoichiometric quantities of Wilkinson's catalyst
Wilkinson's catalyst (chloridotris(triphenylphosphine)rhodium(I)) is a coordination complex of rhodium with the formula hCl(PPh3)3 where 'Ph' denotes a phenyl group. It is a red-brown colored solid that is soluble in hydrocarbon solv ...
was able to affect the same reaction at lower temperatures, now known as the Tsuji–Wilkinson decarbonylation reaction.
 His pioneering work resulting in the development of the
His pioneering work resulting in the development of the Tsuji–Trost reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coor ...
was the discovery that allyl-PdII complexes react with malonates, acetoacetates, and enamines yielding alpha-allylated carbonyl compounds, which was later generalized and rendered asymmetric by Barry Trost
Barry M. Trost (born June 13, 1941, in Philadelphia) is an American chemist who is the Job and Gertrud Tamaki Professor Emeritus in the School of Humanities and Sciences at Stanford University. The Tsuji–Trost reaction and the Trost ligand ar ...
. Tsuji additionally noted the utility of these alpha-allylated ketones and esters in preparing 1,4-dicarbonyl compounds using the Wacker oxidation
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) is an industrial chemical reaction: the aerobic oxidation of ethylene to acetaldehyde in the presence of catalysis, catalytic, aqueous palladium( ...
.
 Later research hinged on the discovery that allyl acetoacetates decarboxylate and regioselectively ''alpha''-allylate in the presence of catalytic Pd(OAc)2 and PPh3 ''via'' the intermediacy of an allylpalladium enolate - a catalytic
Later research hinged on the discovery that allyl acetoacetates decarboxylate and regioselectively ''alpha''-allylate in the presence of catalytic Pd(OAc)2 and PPh3 ''via'' the intermediacy of an allylpalladium enolate - a catalytic Carroll rearrangement
The Carroll rearrangement is a rearrangement reaction in organic chemistry and involves the transformation of a β-ketone, keto allyl ester into a α-allyl-β-ketocarboxylic acid. This organic reaction is accompanied by decarboxylation and the fina ...
. Following work revealed that replacement of PPh3 in the reaction mixture with the bidentate ligand dppe DPPE may refer to the chemicals:
* 1,2-Bis(diphenylphosphino)ethane
* Dipalmitoylphosphatidylethanolamine
{{disambiguation ...
could favor ''beta''-hydride elimination of the palladium enolate, yielding enones. This transformation was applied by Tsuji to the racemic synthesis of methyl jasmonate
Methyl jasmonate (abbreviated MeJA) is a volatile organic compound used in plant defense and many diverse developmental pathways such as seed germination, root growth, flowering, fruit ripening, and senescence. Methyl jasmonate is derived from ja ...
. Tsuji also found that the palladium enolate could be engaged in intramolecular aldol reactions.
Awards
* 1980 - Chemical Society of Japan Award, for "research on new organic synthesis processes using transition metal compounds" * 1994 - Purple ribbon medal of honor * 2004 - Japan Academy Prize, for "research on new organic synthesis reactions using palladium catalysts" * 2014 -Tetrahedron Prize The Tetrahedron Prize for Creativity in Organic Chemistry or Bioorganic and Medicinal Chemistry is awarded annually by Elsevier, the publisher of Tetrahedron Publications. It was established in 1980 and named in honour of the founding co-chairmen o ...
* 2016 - Asian Scientist 100
The Asian Scientist 100 is an annually published list of 100 prize-winning Asian researchers, academicians, innovators and business leaders from across the Asia-Pacific region and a range of scientific disciplines. Recipients "must have received ...
, ''Asian Scientist
''Asian Scientist'' is an English language science and technology magazine published in Singapore.
History and profile
''Asian Scientist'' was launched as a blog in March 2011 by Juliana Chan. The blog's popularity eventually led to a partnersh ...
''
References
{{DEFAULTSORT:Tsuji, Jiro 1927 births 2022 deaths 20th-century Japanese chemists Kyoto University alumni People from Shiga Prefecture Scientists from Shiga Prefecture