Jacobsen's catalyst on:
[Wikipedia]
[Google]
[Amazon]
Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a
 After the addition of the oxidant to the system, O=Mn(V) is generally accepted to be the active oxidant species formed (step A). The substrate is thought to approach the metal- oxo bond from the side at a perpendicular orientation in relation to the catalyst in order to allow favorable orbital overlap. This mechanism, which was originally proposed by John Groves to explain porphyrin-catalyzed epoxidation reactions, is commonly referred to as a "side-on perpendicular approach". The approach is over the diamine bridge, where the steric bulk of the tert-butyl groups on the periphery of the ligand do not interfere with the alkene's approach (see below). However, as is the case with the overall mechanism, the pathway of alkene approach is also debated.
After the addition of the oxidant to the system, O=Mn(V) is generally accepted to be the active oxidant species formed (step A). The substrate is thought to approach the metal- oxo bond from the side at a perpendicular orientation in relation to the catalyst in order to allow favorable orbital overlap. This mechanism, which was originally proposed by John Groves to explain porphyrin-catalyzed epoxidation reactions, is commonly referred to as a "side-on perpendicular approach". The approach is over the diamine bridge, where the steric bulk of the tert-butyl groups on the periphery of the ligand do not interfere with the alkene's approach (see below). However, as is the case with the overall mechanism, the pathway of alkene approach is also debated.
 The ease with which Jacobsen's catalyst selectively epoxidizes cis-alkenes has been difficult to replicate with terminal and trans-alkenes. Structural changes to the ligand and adaptations to the protocol for the epoxidation reaction, however, have led to some successes in these areas. For example, derivatives of Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the terminal alkene
The ease with which Jacobsen's catalyst selectively epoxidizes cis-alkenes has been difficult to replicate with terminal and trans-alkenes. Structural changes to the ligand and adaptations to the protocol for the epoxidation reaction, however, have led to some successes in these areas. For example, derivatives of Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the terminal alkene
coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
of manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
and a salen-type ligand A metal salen complex is a coordination compound between a metal cation and a ligand derived from ''N'',''N''′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt , usua ...
. It is used as an asymmetric catalyst
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
in the Jacobsen epoxidation
The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless e ...
, which is renowned for its ability to enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
ly transform prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
If two identical substituents are attach ...
alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed fro ...
. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
Enantiomerically pure epoxides are desirable as building blocks for complex molecules with specific chirality. Biologically active compounds can exhibit radically different activity based on differences in chirality and therefore the ability to obtain desired stereocenters in a molecule is of great importance to the pharmaceutical industry. Jacobsen's catalyst and other asymmetric catalysts are particularly useful in this field; for example, Jacobsen's catalyst was used to synthesize phenylisoserine, a side chain to the famous anti-cancer drug Taxol
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer. This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cance ...
, in a four-step synthesis as early as 1992.
Structure and basic properties
Jacobsen's catalyst consists of asalen ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff base ...
, tetradentate, meaning it binds to the central manganese metal through four bonds, one to each oxygen and nitrogen atom of the salen backbone. Its chirality is conferred from the diamine-derived backbone. The aryl groups are decorated with tert-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, givi ...
substituents, which amplify the asymmetry around the Mn center.
Preparation
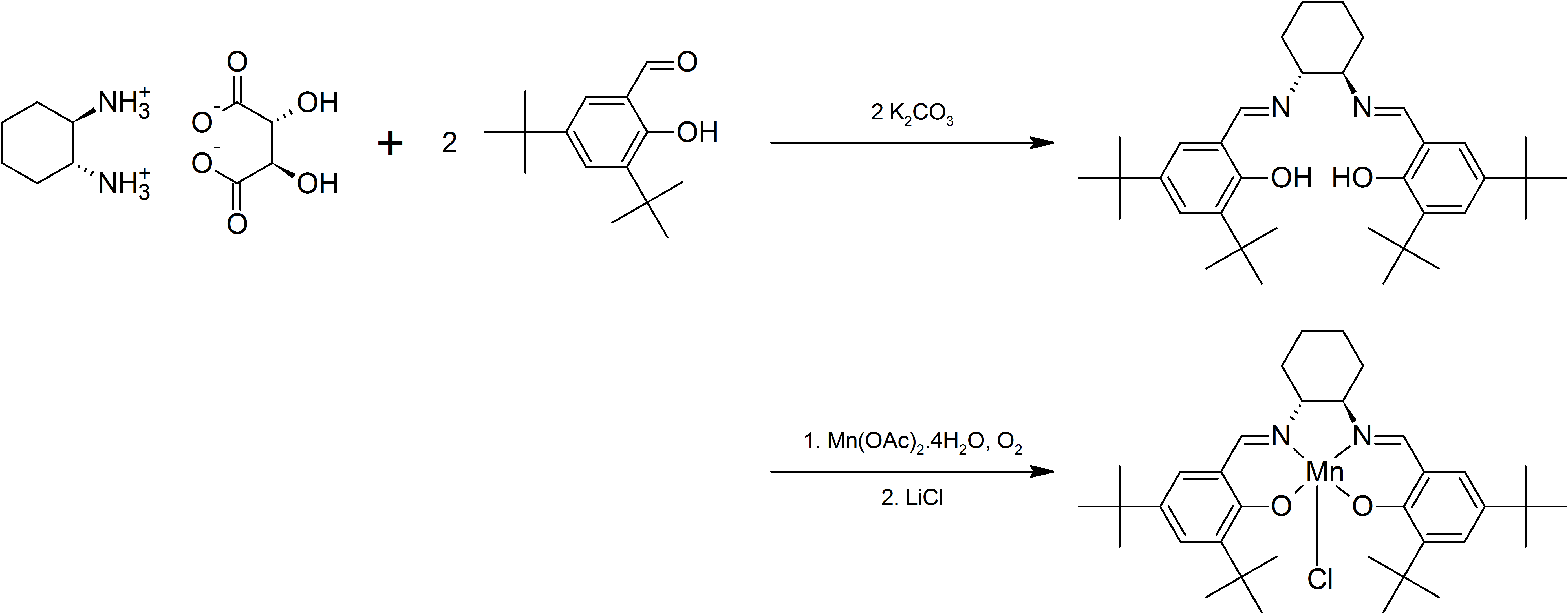
Both enantiomers of Jacobsen's catalyst are commercially available. Jacobsen's catalyst can be prepared by separating 1,2-diaminocyclohexane into its component enantiomers and then reacting the appropriatetartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−.
The main forms of tartrates used commercially are pure crystalline ta ...
with 3,5-di-tert-butyl-2-hydroxybenzaldehyde to form a Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
(see intermediate formed in the reaction scheme below). Reaction with manganese(II) acetate in the presence of air gives the manganese(III) complex, which may be isolated as the chloro derivative after the addition of lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlor ...
. Shown below is the preparation of the (R,R)-enantiomer. The synthesis has been adapted for undergraduate level chemistry courses in order to stress the importance of enantiomerically pure compounds.
:
Reaction mechanism
In general, two mechanisms have been suggested. Because Jacobsen's catalyst epoxidizes conjugated alkenes (i.e. those in which there are multiple double bonds on alternating carbons) most effectively, the generally accepted mechanism is based on a radical intermediate which is stabilized due to the conjugated nature of the substrate. For non-conjugated alkenes, the substrate is far less able to stabilize a radical, making a radical intermediate more unlikely. In this case, a concerted mechanism in which the bond to the oxygen is simultaneously broken with metal-center while it is formed with the substrate is probable. However, more recent studies have indicated a radical intermediate is possible, challenging the assumption that non-conjugated alkenes undergo concerted mechanisms. In the original catalytic reaction, iodosylarenes (PhIO) were used as thestoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
, but soon after it was found that chlorine bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
(NaClO), a cheaper alternative, works as well. While other oxidants subsequently have been used, bleach continues to be the most common.
 The ease with which Jacobsen's catalyst selectively epoxidizes cis-alkenes has been difficult to replicate with terminal and trans-alkenes. Structural changes to the ligand and adaptations to the protocol for the epoxidation reaction, however, have led to some successes in these areas. For example, derivatives of Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the terminal alkene
The ease with which Jacobsen's catalyst selectively epoxidizes cis-alkenes has been difficult to replicate with terminal and trans-alkenes. Structural changes to the ligand and adaptations to the protocol for the epoxidation reaction, however, have led to some successes in these areas. For example, derivatives of Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the terminal alkene styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
. The low temperature of the reaction favors only one pathway, the cis pathway, while m-CPBA is used because of water's high freezing point. Little success has occurred with the epoxidation of trans alkenes by manganese compounds but other salen coordination compounds, such as oxochromium complexes, can be used.
Variations
The ligand structure of Jacobsen's catalyst is easily modified for use over a wide range of reactions, such as epoxide-ring openings, Diels-Alder reactions, and conjugate additions. For example, an analogous catalyst with an aluminum metal center has been used for thecarbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
of epoxides in order to obtain beta-lactones
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
.
See also
*Asymmetric catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
*Enantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
*Jacobsen epoxidation
The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless e ...
*Salen ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff base ...
References
{{reflist Manganese(III) compounds Catalysis Metal salen complexes Tert-butyl compounds Catalysts