intramolecular reaction on:
[Wikipedia]
[Google]
[Amazon]
Intramolecular in
 For the
For the
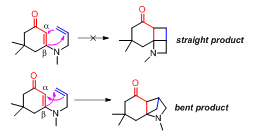
 The length of the tether affects the stereochemical outcome of the +2reaction. Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the -carbon of the enone. When the tether consists only two carbons, the “bent” product is generated where the -carbon of the enone is connected to the terminal carbon of the alkene (Figure 2).
The length of the tether affects the stereochemical outcome of the +2reaction. Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the -carbon of the enone. When the tether consists only two carbons, the “bent” product is generated where the -carbon of the enone is connected to the terminal carbon of the alkene (Figure 2).
 Tethered +2reactions have been used to synthesize organic compounds with interesting ring systems and topologies. For example, +2photocyclization was used to construct the tricyclic core structure in
Tethered +2reactions have been used to synthesize organic compounds with interesting ring systems and topologies. For example, +2photocyclization was used to construct the tricyclic core structure in 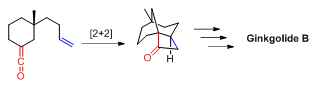
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
describes a process or characteristic limited within the structure of a single molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
, a property or phenomenon limited to the extent of a single molecule.
Examples
* intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecularorganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
s, two reaction sites are contained within a single molecule. This creates a very high effective concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', ''number concentration'', ...
(resulting in high reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per uni ...
s), and, therefore, many intramolecular reactions that would not occur as an intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
reaction between two compounds take place.
Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensat ...
and the Madelung synthesis
The Madelung synthesis is a chemical reaction that produces (substituted or unsubstituted) indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature. The Madelung synthesis was reported in 1912 by Walter Ma ...
.
Relative rates
Intramolecular reactions, especially ones leading to the formation of 5- and 6-membered rings, are rapid compared to an analogous intermolecular process. This is largely a consequence of the reduced entropic cost for reaching the transition state of ring formation and the absence of significant strain associated with formation of rings of these sizes. For the formation of different ring sizes via cyclization of substrates of varying tether length, the order of reaction rates (rate constants ''kn'' for the formation of an ''n''-membered ring) is usually ''k''5 > ''k''6 > ''k''3 > ''k''7 > ''k''4 as shown below for a series of ω-bromoalkylamines. This somewhat complicated rate trend reflects the interplay of these entropic and strain factors: For the
For the angle strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are ...
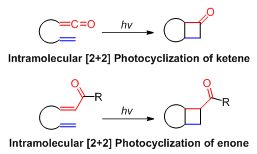
experienced at the transition state. Although three-membered rings are more strained, formation of aziridine is faster than formation of azetidine due to the proximity of the leaving group and nucleophile in the former, which increases the probability that they would meet in a reactive conformation. The same reasoning holds for the Tethered intramolecular +2reactions
Tethered intramolecular +2reactions entail the formation ofcyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerci ...
and cyclobutanone
Cyclobutanone is an organic compound with molecular formula (CH2)3CO. It is a four-membered cyclic ketone (cycloalkanone). It is a colorless volatile liquid at room temperature. Since cyclopropanone is highly sensitive, cyclobutanone is the sm ...
via intramolecular 2+2 photocycloadditions. Tethering ensures formation of a multi-cyclic system.
 The length of the tether affects the stereochemical outcome of the +2reaction. Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the -carbon of the enone. When the tether consists only two carbons, the “bent” product is generated where the -carbon of the enone is connected to the terminal carbon of the alkene (Figure 2).
The length of the tether affects the stereochemical outcome of the +2reaction. Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the -carbon of the enone. When the tether consists only two carbons, the “bent” product is generated where the -carbon of the enone is connected to the terminal carbon of the alkene (Figure 2).
 Tethered +2reactions have been used to synthesize organic compounds with interesting ring systems and topologies. For example, +2photocyclization was used to construct the tricyclic core structure in
Tethered +2reactions have been used to synthesize organic compounds with interesting ring systems and topologies. For example, +2photocyclization was used to construct the tricyclic core structure in ginkgolide
Ginkgolides are biologically active terpenic lactones present in ''Ginkgo biloba''. They are diterpenoids with 20-carbon skeletons, which are biosynthesized from geranylgeranyl pyrophosphate.
Examples
Ginkgolide B
Ginkgolide B, specificall ...
B by E. J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many a ...
and co-workers in 1988.

Molecular tethers
In a niche concept called molecular tethers, otherwise-intermolecular reactions can be made temporarily intramolecular by anchoring both reactions by atether
A tether is a cord, fixture, or flexible attachment that characteristically anchors something movable to something fixed; it also maybe used to connect two movable objects, such as an item being towed by its tow.
Applications for tethers includ ...
with all the advantages associated to it. Popular choices of tether contain a carbonate ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and the ...
, boronic ester, silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting ...
, or a silyl acetal link (silicon tethers) which are fairly inert in many organic reactions yet can be cleaved by specific reagents. The main hurdle for this strategy to work is selecting the proper length for the tether and making sure reactive groups have an optimal orientation with respect to each other. An examples is a Pauson–Khand reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a 2+2+1.html" ;"title="/nowiki>2+2+1">/nowiki>2+2+1/nowiki> cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone ...
of an alkene and an alkyne tethered together via a silyl ether.
In this particular reaction, the tether angle bringing the reactive groups together is effectively reduced by placing isopropyl groups on the silicon atom via the Thorpe–Ingold effect. No reaction takes place when these bulky groups are replaced by smaller methyl groups.
Another example is a photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
+2 ycloaddition with two alkene groups tethered through a silicon acetal group (racemic, the other enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
not depicted), which is subsequently cleaved by TBAF
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tet ...
yielding the endo-diol.
:
Without the tether, the exo isomer
In organic chemistry, ''endo''–''exo'' isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The prefix ''endo'' is reserved for the isomer with the substituent located closest, ...
forms.
References
{{DEFAULTSORT:Intramolecular Reaction Reaction mechanisms Molecular physics Organic chemistry