Isomigrastatin on:
[Wikipedia]
[Google]
[Amazon]
Isomigrastatin is an analogue of
 Fragment coupling of intermediate 11 and 15 would be the next step. Using lithium borohydride, lactol arrangement of reagent 11 is reduced to create alcohol 16 which could be converted to 17 as well. Coupled with phosphorane 15, reagent 16 and 17 are oxidized to synthesize aldehyde 18.
Fragment coupling of intermediate 11 and 15 would be the next step. Using lithium borohydride, lactol arrangement of reagent 11 is reduced to create alcohol 16 which could be converted to 17 as well. Coupled with phosphorane 15, reagent 16 and 17 are oxidized to synthesize aldehyde 18.
 Enone 18 is then reduced by (S)-Me-CBS Corey catalyst to make intermediate 19, and lithium cyanomethylcuprate is added to make intermediate 21.
Enone 18 is then reduced by (S)-Me-CBS Corey catalyst to make intermediate 19, and lithium cyanomethylcuprate is added to make intermediate 21.
 Acylation of alcohol 21 with racemic selenoacid 23 then leads to be intermediate 24, and ring-closing metathesis of 24 causes intermediate 26. Then it finally affords isomigrastatin by oxidative deselenation.
Acylation of alcohol 21 with racemic selenoacid 23 then leads to be intermediate 24, and ring-closing metathesis of 24 causes intermediate 26. Then it finally affords isomigrastatin by oxidative deselenation.

migrastatin
Migrastatin is an organic compound which naturally occurs in the '' Streptomyces platensis'' bacteria. Migrastatin and several of its analogues (including Isomigrastatin) have shown to have potential in treating cancer, as it inhibits the metastas ...
, an organic compound that naturally occurs in the '' Streptomyces platensis'' bacteria. Isomigrastatin has shown promise as a drug in the treatment of cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
. A laboratory synthesis was reported in 2007.Synthesis
Total synthesis
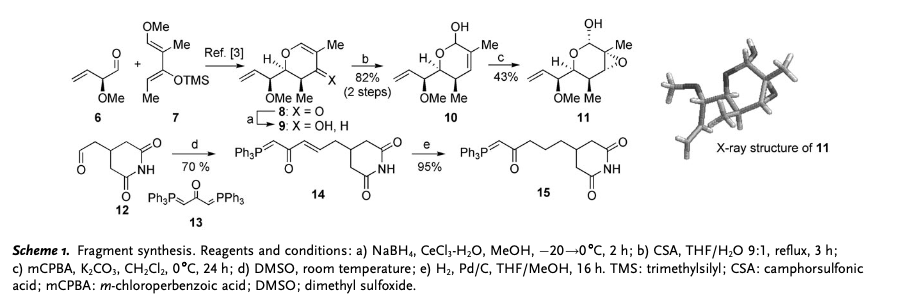
Migrastatin synthesis is a precursor of isomigrastatin. In order to synthesize isomigrastatin, reagent 11 and 15 need to be prepared. Through LACDAC reaction, Luche reduction, aqueous Ferrier rearrangement and Epoxidation, reagent 6, 7, 8, 9, 10 are synthesized to 11. Aldehyde 12 reacts is alkyldenated by Witting reagent 13 to make 14, and 14 is hydrogenated to afford 15. Fragment coupling of intermediate 11 and 15 would be the next step. Using lithium borohydride, lactol arrangement of reagent 11 is reduced to create alcohol 16 which could be converted to 17 as well. Coupled with phosphorane 15, reagent 16 and 17 are oxidized to synthesize aldehyde 18.
Fragment coupling of intermediate 11 and 15 would be the next step. Using lithium borohydride, lactol arrangement of reagent 11 is reduced to create alcohol 16 which could be converted to 17 as well. Coupled with phosphorane 15, reagent 16 and 17 are oxidized to synthesize aldehyde 18.
 Enone 18 is then reduced by (S)-Me-CBS Corey catalyst to make intermediate 19, and lithium cyanomethylcuprate is added to make intermediate 21.
Enone 18 is then reduced by (S)-Me-CBS Corey catalyst to make intermediate 19, and lithium cyanomethylcuprate is added to make intermediate 21.
 Acylation of alcohol 21 with racemic selenoacid 23 then leads to be intermediate 24, and ring-closing metathesis of 24 causes intermediate 26. Then it finally affords isomigrastatin by oxidative deselenation.
Acylation of alcohol 21 with racemic selenoacid 23 then leads to be intermediate 24, and ring-closing metathesis of 24 causes intermediate 26. Then it finally affords isomigrastatin by oxidative deselenation.

Biosynthesis
In terms of natural product, isomigrastatin is the polyketide that contains the glutarimide.Ma, M., Kwong, T., Lim, S. K., Ju, J., Lohman, J. R., & Shen, B. (2013). Post-polyketide synthase steps in iso-migrastatin biosynthesis, featuring tailoring enzymes with broad substrate specificity. Journal of the American Chemical Society, 135(7), 2489–2492. https://doi.org/10.1021/ja4002635 Biosynthesis of isomigrastatin starts with the PKS product 10 which is derived from S.platensis. PKS product that is lack of methyltransferase domain in module-5, a ketoreductase domain in module-8, and a KR and an enoylreductase domain in module-10 is needed to synthesize intermediate for isomigrastatin. On top of that, four tailoring steps are followed through intermediates. First, hydroxylation at C-8. Second, O-methylation at OHC-8. Third, dehydration at C-17 OH. Last, C-16 and C-17 olefin is reduced. PKS product 10 is then isolated to isomigrastatin.References
Macrolides Glutarimides Secondary alcohols Ethers {{alcohol-stub