isoalloxazine on:
[Wikipedia]
[Google]
[Amazon]
Isoalloxazine is the structural foundation of 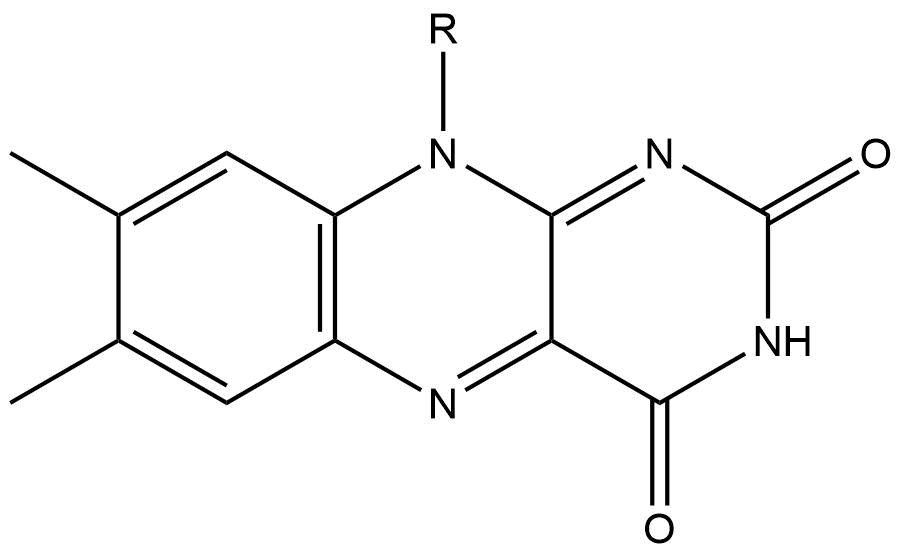

flavins
Flavins (from Latin ''flavus'', "yellow") are organic compounds, like their base, pteridine. They are formed by the tricyclic heterocycle isoalloxazine. The biochemical source is the vitamin riboflavin. The flavin moiety is often attached with a ...
such as riboflavin
Riboflavin, also known as vitamin B2, is a vitamin found in food and sold as a dietary supplement. It is essential to the formation of two major coenzymes, flavin mononucleotide and flavin adenine dinucleotide. These coenzymes are involved in ...
(vitamin B2) and is a heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
. It has a tricyclic
Tricyclics are chemical compounds that contain three interconnected rings of atoms.
Many compounds have a tricyclic structure, but in pharmacology, the term has traditionally been reserved to describe heterocyclic drugs. Among these are antid ...
structure which means it has three interconnected rings of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s and is a tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
of alloxazine. The structure is formed by primary-secondary aromatic o-diamines and they are a high-melting crystalline substance. The R-group is used to attach various flavin groups It has a similar structure to pteridines
Pteridine is an aromatic chemical compound composed of fused pyrimidine and pyrazine rings. A pteridine is also a group of heterocyclic compounds containing a wide variety of substitutions on this structure. Pterins and flavins are classes of su ...
which has two interconnected rings. Isoalloxazine was first obtained in 1934 by Richard Kuhn
Richard Johann Kuhn (; 3 December 1900 – 1 August 1967) was an Austrian-German biochemist who was awarded the Nobel Prize in Chemistry in 1938 "for his work on carotenoids and vitamins".
Biography
Early life
Kuhn was born in Vienna, Austria ...
an Austrian-German biochemist and lab mates. 
Isoalloxazine ring
Isoalloxazine rings can exist in differentredox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
and ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
states depending on the chemistry of FMN and FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
associated with it. Using the redox-active isoalloxazine system, FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
and FMN are able to do one and two electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
transfer reactions and also be coupled with proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
transfers

References
{{reflist Heterocyclic compounds with 3 rings Nitrogen heterocycles Pteridines