Iron In Biology on:
[Wikipedia]
[Google]
[Amazon]
 Iron is an important biological element. It is used in both the ubiquitous
Iron is an important biological element. It is used in both the ubiquitous

 Examples of iron-containing proteins in higher organisms include hemoglobin,
Examples of iron-containing proteins in higher organisms include hemoglobin,

 Most well-nourished people in industrialized countries have 4 to 5 grams of iron in their bodies (∼38 mg iron/kg body weight for women and ∼50 mg iron/kg body for men). Of this, about is contained in the hemoglobin needed to carry oxygen through the blood (around 0.5 mg of iron per mL of blood), and most of the rest (approximately 2 grams in adult men, and somewhat less in women of childbearing age) is contained in
Most well-nourished people in industrialized countries have 4 to 5 grams of iron in their bodies (∼38 mg iron/kg body weight for women and ∼50 mg iron/kg body for men). Of this, about is contained in the hemoglobin needed to carry oxygen through the blood (around 0.5 mg of iron per mL of blood), and most of the rest (approximately 2 grams in adult men, and somewhat less in women of childbearing age) is contained in
 Iron is an important biological element. It is used in both the ubiquitous
Iron is an important biological element. It is used in both the ubiquitous Iron-sulfur protein
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clu ...
s and in Vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () ( chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, ...
s it is used in Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
which is essential for Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the c ...
and oxygen transport.
Overview
Iron is required for life. electronic-book Theiron–sulfur cluster
Iron–sulfur clusters (or iron–sulphur clusters in British spelling) are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe– ...
s are pervasive and include nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the Organic redox reaction, reduction of nitrogen (N2) to ammonia (NH3). Nitrog ...
, the enzymes responsible for biological nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
. Iron-containing proteins participate in transport, storage and used of oxygen. Iron proteins are involved in electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons.
Electrochemical processes ar ...
. The ubiquity of Iron in life has led to the Iron–sulfur world hypothesis
The iron–sulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry, w ...
that Iron was a central component of the environment of early life.

cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of bin ...
(see high-valent iron
High-valent iron commonly denotes compounds and intermediates in which iron is found in a formal oxidation state > 3 that show a number of bonds > 6 with a coordination number ≤ 6. The term is rather uncommon for hepta-coordinate compounds of ir ...
), and catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
. The average adult human contains about 0.005% body weight of iron, or about four grams, of which three quarters is in hemoglobin – a level that remains constant despite only about one milligram of iron being absorbed each day, because the human body recycles its hemoglobin for the iron content.
Microbial growth may be assisted by oxidation of iron(II) or by reduction of iron (III).
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric iron is poorly soluble near neutral pH. Thus, these organisms have developed means to absorb iron as complexes, sometimes taking up ferrous iron before oxidising it back to ferric iron. In particular, bacteria have evolved very high-affinity sequestering agents calledsiderophore
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is no ...
s.
After uptake in human cells, iron storage is precisely regulated. A major component of this regulation is the protein transferrin
Transferrins are glycoproteins found in vertebrates which bind to and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encode ...
, which binds iron ions absorbed from the duodenum
The duodenum is the first section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear, and the terms anterior intestine or proximal intestine m ...
and carries it in the blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the c ...
to cells. Transferrin contains Fe3+ in the middle of a distorted octahedron, bonded to one nitrogen, three oxygens and a chelating carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
anion that traps the Fe3+ ion: it has such a high stability constant that it is very effective at taking up Fe3+ ions even from the most stable complexes. At the bone marrow, transferrin is reduced from Fe3+ and Fe2+ and stored as ferritin
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary ' ...
to be incorporated into hemoglobin.
The most commonly known and studied bioinorganic
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those t ...
iron compounds (biological iron molecules) are the heme proteins
A hemeprotein (or haemprotein; also hemoprotein or haemoprotein), or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen ...
: examples are hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
, myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
, and cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
. These compounds participate in transporting gases, building enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
, and transferring electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s.Greenwood and Earnshaw, pp. 1098–104 Metalloproteins
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains al ...
are a group of proteins with metal ion cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
. Some examples of iron metalloproteins are ferritin
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary ' ...
and rubredoxin
Rubredoxins are a class of low-molecular-weight iron-containing proteins found in sulfur-metabolizing bacteria and archaea. Sometimes rubredoxins are classified as iron-sulfur proteins; however, in contrast to iron-sulfur proteins, rubredoxins d ...
. Many enzymes vital to life contain iron, such as catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
, lipoxygenases, and IRE-BP
The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR
, bind to iron-responsive elements (IREs) in the regulation of human iron metabolism.
Function
ACO1, or IRP1, is a bifunctional protein that functions as a ...
.
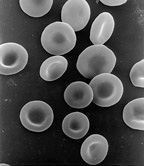
Hemoglobin is an oxygen carrier that occurs in red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
, which stores it until it is needed for the metabolic oxidation of glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
, generating energy. Here the hemoglobin binds to carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
anions) back to the lungs where it is exhaled. In hemoglobin, the iron is in one of four heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
groups and has six possible coordination sites; four are occupied by nitrogen atoms in a porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
ring, the fifth by an imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers ...
nitrogen in a histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the de ...
residue of one of the protein chains attached to the heme group, and the sixth is reserved for the oxygen molecule it can reversibly bind to. When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
group (in the hydrophobic protein interior) is in a high-spin configuration. It is thus too large to fit inside the porphyrin ring, which bends instead into a dome with the Fe2+ ion about 55 picometers above it. In this configuration, the sixth coordination site reserved for the oxygen is blocked by another histidine residue.
When deoxyhemoglobin picks up an oxygen molecule, this histidine residue moves away and returns once the oxygen is securely attached to form a hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
with it. This results in the Fe2+ ion switching to a low-spin configuration, resulting in a 20% decrease in ionic radius so that now it can fit into the porphyrin ring, which becomes planar. (Additionally, this hydrogen bonding results in the tilting of the oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O2–Fe bridges that would lead to electron transfer, the oxidation of Fe2+ to Fe3+, and the destruction of hemoglobin.) This results in a movement of all the protein chains that leads to the other subunits of hemoglobin changing shape to a form with larger oxygen affinity. Thus, when deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases, and vice versa. Myoglobin, on the other hand, contains only one heme group and hence this cooperative effect cannot occur. Thus, while hemoglobin is almost saturated with oxygen in the high partial pressures of oxygen found in the lungs, its affinity for oxygen is much lower than that of myoglobin, which oxygenates even at low partial pressures of oxygen found in muscle tissue. As described by the Bohr effect (named after Christian Bohr
Christian Harald Lauritz Peter Emil Bohr (1855–1911) was a Danish physician, father of the physicist and Nobel laureate Niels Bohr, as well as the mathematician and football player Harald Bohr and grandfather of another physicist and Nobel lau ...
, the father of Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. B ...
), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.

Carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and phosphorus trifluoride
Phosphorus trifluoride (formula P F3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed ...
are poisonous to humans because they bind to hemoglobin similarly to oxygen, but with much more strength, so that oxygen can no longer be transported throughout the body. Hemoglobin bound to carbon monoxide is known as carboxyhemoglobin
Carboxyhemoglobin (carboxyhaemoglobin BrE) (symbol COHb or HbCO) is a stable complex of carbon monoxide and hemoglobin (Hb) that forms in red blood cells upon contact with carbon monoxide. Carboxyhemoglobin is often mistaken for the compound form ...
. This effect also plays a minor role in the toxicity of cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
, but there the major effect is by far its interference with the proper functioning of the electron transport protein cytochrome a. The cytochrome proteins also involve heme groups and are involved in the metabolic oxidation of glucose by oxygen. The sixth coordination site is then occupied by either another imidazole nitrogen or a methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ro ...
sulfur, so that these proteins are largely inert to oxygen – with the exception of cytochrome a, which bonds directly to oxygen and thus is very easily poisoned by cyanide. Here, the electron transfer takes place as the iron remains in low spin but changes between the +2 and +3 oxidation states. Since the reduction potential of each step is slightly greater than the previous one, the energy is released step-by-step and can thus be stored in adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of ...
. Cytochrome a is slightly distinct, as it occurs at the mitochondrial membrane, binds directly to oxygen, and transports protons as well as electrons, as follows:
:4 Cytc2+ + O2 + 8H → 4 Cytc3+ + 2 H2O + 4H
Although the heme proteins are the most important class of iron-containing proteins, the iron–sulfur protein
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clu ...
s are also very important, being involved in electron transfer, which is possible since iron can exist stably in either the +2 or +3 oxidation states. These have one, two, four, or eight iron atoms that are each approximately tetrahedrally coordinated to four sulfur atoms; because of this tetrahedral coordination, they always have high-spin iron. The simplest of such compounds is rubredoxin
Rubredoxins are a class of low-molecular-weight iron-containing proteins found in sulfur-metabolizing bacteria and archaea. Sometimes rubredoxins are classified as iron-sulfur proteins; however, in contrast to iron-sulfur proteins, rubredoxins d ...
, which has only one iron atom coordinated to four sulfur atoms from cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
residues in the surrounding peptide chains. Another important class of iron–sulfur proteins is the ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
s, which have multiple iron atoms. Transferrin does not belong to either of these classes.
The ability of sea mussel
Mussel () is the common name used for members of several families of bivalve molluscs, from saltwater and Freshwater bivalve, freshwater habitats. These groups have in common a shell whose outline is elongated and asymmetrical compared with other ...
s to maintain their grip on rocks in the ocean is facilitated by their use of organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
iron-based bonds in their protein-rich cuticle
A cuticle (), or cuticula, is any of a variety of tough but flexible, non-mineral outer coverings of an organism, or parts of an organism, that provide protection. Various types of "cuticle" are non- homologous, differing in their origin, structu ...
s. Based on synthetic replicas, the presence of iron in these structures increased elastic modulus
An elastic modulus (also known as modulus of elasticity) is the unit of measurement of an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. The elastic modulus of an object is ...
770 times, tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
58 times, and toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
the Oxygen transport protein.
Human body iron stores
ferritin
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary ' ...
complexes that are present in all cells, but most common in bone marrow, liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
, and spleen
The spleen is an organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter. The word spleen comes .
. The liver stores of ferritin are the primary physiologic source of reserve iron in the body. The reserves of iron in industrialized countries tend to be lower in children and women of child-bearing age than in men and in the elderly. Women who must use their stores to compensate for iron lost through menstruation
Menstruation (also known as a period, among other colloquial terms) is the regular discharge of blood and mucosal tissue from the inner lining of the uterus through the vagina. The menstrual cycle is characterized by the rise and fall of hor ...
, pregnancy
Pregnancy is the time during which one or more offspring develops ( gestates) inside a woman's uterus (womb). A multiple pregnancy involves more than one offspring, such as with twins.
Pregnancy usually occurs by sexual intercourse, but ca ...
or lactation
Lactation describes the secretion of milk from the mammary glands and the period of time that a mother lactates to feed her young. The process naturally occurs with all sexually mature female mammals, although it may predate mammals. The proces ...
have lower non-hemoglobin body stores, which may consist of , or even less.
Of the body's total iron content, about is devoted to cellular proteins that use iron for important cellular processes like storing oxygen (myoglobin) or performing energy-producing redox reactions (cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of bin ...
s). A relatively small amount (3–4 mg) circulates through the plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
, bound to transferrin. Because of its toxicity, free soluble iron is kept in low concentration in the body.
Iron deficiency
Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key ...
first affects the storage of iron in the body, and depletion of these stores is thought to be relatively asymptomatic, although some vague and non-specific symptom
Signs and symptoms are the observed or detectable signs, and experienced symptoms of an illness, injury, or condition. A sign for example may be a higher or lower temperature than normal, raised or lowered blood pressure or an abnormality showin ...
s have been associated with it. Since iron is primarily required for hemoglobin, iron deficiency anemia
Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, s ...
is the primary clinical manifestation of iron deficiency. Iron-deficient people will suffer or die from organ damage well before their cells run out of the iron needed for intracellular processes like electron transport.
Macrophages
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer ce ...
of the reticuloendothelial system
In anatomy the term "reticuloendothelial system" (abbreviated RES), often associated nowadays with the mononuclear phagocyte system (MPS), was originally launched by the beginning of the 20th century to denote a system of specialised cells that eff ...
store iron as part of the process of breaking down and processing hemoglobin from engulfed red blood cells. Iron is also stored as a pigment called hemosiderin
Hemosiderin image of a kidney viewed under a microscope. The brown areas represent hemosiderin
Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives rise ...
, which is an ill-defined deposit of protein and iron, created by macrophages where excess iron is present, either locally or systemically, e.g., among people with iron overload due to frequent blood cell destruction and the necessary transfusions their condition calls for. If systemic iron overload is corrected, over time the hemosiderin is slowly resorbed by the macrophages.
Mechanisms of iron regulation
Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron byenterocytes
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its s ...
, the cells that line the interior of the intestines
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans ...
, and the uncontrolled loss of iron from epithelial sloughing, sweat, injuries and blood loss. In addition, systemic iron is continuously recycled. Cellular iron levels are controlled differently by different cell types due to the expression of particular iron regulatory and transport proteins.
Systemic iron regulation

=Dietary iron uptake
= The absorption of dietary iron is a variable and dynamic process. The amount of iron absorbed compared to the amount ingested is typically low, but may range from 5% to as much as 35% depending on circumstances and type of iron. The efficiency with which iron is absorbed varies depending on the source. Generally, the best-absorbed forms of iron come from animal products. Absorption of dietary iron in iron salt form (as in most supplements) varies somewhat according to the body's need for iron, and is usually between 10% and 20% of iron intake. Absorption of iron from animal products, and some plant products, is in the form of heme iron, and is more efficient, allowing absorption of from 15% to 35% of intake. Heme iron in animals is from blood and heme-containing proteins in meat and mitochondria, whereas in plants, heme iron is present in mitochondria in all cells that use oxygen for respiration. Like most mineral nutrients, the majority of the iron absorbed from digested food or supplements is absorbed in theduodenum
The duodenum is the first section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear, and the terms anterior intestine or proximal intestine m ...
by enterocyte
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its ...
s of the duodenal lining. These cells have special molecules that allow them to move iron into the body. To be absorbed, dietary iron can be absorbed as part of a protein such as heme protein or iron must be in its ferrous Fe2+ form. A ferric reductase enzyme on the enterocytes' brush border
A brush border (striated border or brush border membrane) is the microvilli-covered surface of simple cuboidal and simple columnar epithelium found in different parts of the body. Microvilli are approximately 100 nanometers in diameter and the ...
, duodenal cytochrome B (Dcytb
Duodenal cytochrome B (Dcytb) also known as cytochrome b reductase 1 is an enzyme that in humans is encoded by the gene.
Dcytb CYBRD1 was first identified as a ferric reductase enzyme which catalyzes the reduction of Fe3+ to Fe2+ required for d ...
), reduces ferric Fe3+ to Fe2+. A protein called divalent metal transporter 1 (DMT1
Natural resistance-associated macrophage protein 2 (NRAMP 2), also known as divalent metal transporter 1 (DMT1) and divalent cation transporter 1 (DCT1), is a protein that in humans is encoded by the ''SLC11A2'' (solute carrier family 11, member 2) ...
), which can transport several divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
metals across the plasma membrane, then transports iron across the enterocyte's cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
into the cell. If the iron is bound to heme it is instead transported across the apical membrane by heme carrier protein 1 (HCP1).
These intestinal lining cells can then either store the iron as ferritin
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary ' ...
, which is accomplished by Fe2+ binding to apoferritin (in which case the iron will leave the body when the cell dies and is sloughed off into feces
Feces ( or faeces), known colloquially and in slang as poo and poop, are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the large intestine. Feces contain a relati ...
), or the cell can release it into the body via the only known iron exporter in mammals, ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene, and is part of the Ferroportin (Fpn) FamilyTC# 2.A.100. Ferroportin i ...
. Hephaestin
Hephaestin, also known as HEPH, is a protein which in humans is encoded by the ''HEPH'' gene.
Function
Hephaestin is involved in the metabolism and homeostasis of iron and possibly copper. It is a transmembrane copper-dependent ferroxidase resp ...
, a ferroxidase
Ferroxidase also known as Fe(II):oxygen oxidoreductase is an enzyme that catalyzes the oxidization of iron II to iron III:
: 4 Fe2+ + 4 H+ + O2 ⇔ 4 Fe3+ + 2H2O
Examples
Human genes encoding proteins with ferroxidase activity include:
* CP ...
that can oxidize Fe2+ to Fe3+ and is found mainly in the small intestine, helps ferroportin transfer iron across the basolateral end of the intestine cells. In contrast, ferroportin is post-translationally repressed by hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
, a 25-amino acid peptide hormone. The body regulates iron levels by regulating each of these steps. For instance, enterocytes synthesize more Dcytb, DMT1 and ferroportin in response to iron deficiency anemia. Iron absorption from diet is enhanced in the presence of vitamin C and diminished by excess calcium, zinc, or manganese.
The human body's rate of iron absorption appears to respond to a variety of interdependent factors, including total iron stores, the extent to which the bone marrow is producing new red blood cells, the concentration of hemoglobin in the blood, and the oxygen content of the blood. The body also absorbs less iron during times of inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
, in order to deprive bacteria of iron. Recent discoveries demonstrate that hepcidin regulation of ferroportin is responsible for the syndrome of anemia of chronic disease.
=Iron recycling and loss
= Most of the iron in the body is hoarded and recycled by the reticuloendothelial system, which breaks down aged red blood cells. In contrast to iron uptake and recycling, there is no physiologic regulatory mechanism for excreting iron. People lose a small but steady amount by gastrointestinal blood loss, sweating and by shedding cells of the skin and themucosa
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
l lining of the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organ (biology), organs of the digestive syste ...
. The total amount of loss for healthy people in the developed world amounts to an estimated average of a day for men, and 1.5–2 mg a day for women with regular menstrual periods. People with gastrointestinal parasitic infections, more commonly found in developing countries, often lose more. Those who cannot regulate absorption well enough get disorders of iron overload. In these diseases, the toxicity of iron starts overwhelming the body's ability to bind and store it.
Cellular iron regulation
=Iron import
= Most cell types take up iron primarily throughreceptor-mediated endocytosis
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). Thi ...
via transferrin receptor 1
Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the ''TFRC'' gene. TfR1 is required for iron import from transferrin into cells by endocytosis.
Structure and func ...
(TFR1), transferrin receptor 2
Transferrin receptor 2 (TfR2) is a protein that in humans is encoded by the ''TFR2'' gene. This protein is involved in the uptake of transferrin-bound iron into cells by endocytosis, although its role is minor compared to transferrin receptor 1.
F ...
(TFR2) and GAPDH
Glyceraldehyde 3-phosphate dehydrogenase (abbreviated GAPDH) () is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long establishe ...
. TFR1 has a 30-fold higher affinity for transferrin-bound iron than TFR2 and thus is the main player in this process. The higher order multifunctional glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also acts as a transferrin receptor. Transferrin-bound ferric iron is recognized by these transferrin receptors, triggering a conformational change that causes endocytosis. Iron then enters the cytoplasm from the endosome via importer DMT1 after being reduced to its ferrous state by a STEAP family reductase.
Alternatively, iron can enter the cell directly via plasma membrane divalent cation importers such as DMT1 and ZIP14 (Zrt-Irt-like protein 14). Again, iron enters the cytoplasm in the ferrous state after being reduced in the extracellular space by a reductase such as STEAP2, STEAP3 (in red blood cells), Dcytb (in enterocytes) and SDR2.
=The labile iron pool
= In the cytoplasm, ferrous iron is found in a soluble, chelatable state which constitutes the labile iron pool (~0.001 mM). In this pool, iron is thought to be bound to low-mass compounds such as peptides, carboxylates and phosphates, although some might be in a free, hydrated form ( aqua ions). Alternatively, iron ions might be bound to specialized proteins known as metallochaperones. Specifically, poly-r(C)-binding proteinsPCBP1
Poly(rC)-binding protein 1 is a protein that in humans is encoded by the ''PCBP1'' gene.
This intronless gene is thought to have been generated by retrotransposition of a fully processed PCBP-2 mRNA. This gene and PCBP-2 have paralogues (PCBP3 and ...
and PCBP2
Poly(rC)-binding protein 2 is a protein that in humans is encoded by the ''PCBP2'' gene.
Function
The protein encoded by this gene appears to be multifunctional. It along with PCBP-1 and hnRNPK corresponds to the major cellular poly(rC)-binding ...
appear to mediate transfer of free iron to ferritin (for storage) and non-heme iron enzyme In biochemistry, non-heme iron proteins describe families of enzymes that utilize iron at the active site but lack heme cofactors. Iron-sulfur proteins, including those that are enzymes, are not included in this definition.
Some of non-heme iron p ...
s (for use in catalysis). The labile iron pool is potentially toxic due to iron's ability to generate reactive oxygen species. Iron from this pool can be taken up by mitochondria via mitoferrin to synthesize Fe-S clusters and heme groups.
=The storage iron pool
= Iron can be stored in ferritin as ferric iron due to theferroxidase
Ferroxidase also known as Fe(II):oxygen oxidoreductase is an enzyme that catalyzes the oxidization of iron II to iron III:
: 4 Fe2+ + 4 H+ + O2 ⇔ 4 Fe3+ + 2H2O
Examples
Human genes encoding proteins with ferroxidase activity include:
* CP ...
activity of the ferritin heavy chain. Dysfunctional ferritin may accumulate as hemosiderin
Hemosiderin image of a kidney viewed under a microscope. The brown areas represent hemosiderin
Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives rise ...
, which can be problematic in cases of iron overload. The ferritin storage iron pool is much larger than the labile iron pool, ranging in concentration from 0.7 mM to 3.6 mM.
=Iron export
= Iron export occurs in a variety of cell types, includingneuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
s, red blood cells, macrophages and enterocytes. The latter two are especially important since systemic iron levels depend upon them. There is only one known iron exporter, ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene, and is part of the Ferroportin (Fpn) FamilyTC# 2.A.100. Ferroportin i ...
. It transports ferrous iron out of the cell, generally aided by ceruloplasmin
Ceruloplasmin (or caeruloplasmin) is a ferroxidase enzyme that in humans is encoded by the ''CP'' gene.
Ceruloplasmin is the major copper-carrying protein in the blood, and in addition plays a role in iron metabolism. It was first described in 194 ...
and/or hephaestin
Hephaestin, also known as HEPH, is a protein which in humans is encoded by the ''HEPH'' gene.
Function
Hephaestin is involved in the metabolism and homeostasis of iron and possibly copper. It is a transmembrane copper-dependent ferroxidase resp ...
(mostly in enterocytes), which oxidize iron to its ferric state so it can bind ferritin in the extracellular medium. Hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
causes the internalization of ferroportin, decreasing iron export. Besides, hepcidin seems to downregulate both TFR1 and DMT1 through an unknown mechanism. Another player assisting ferroportin in effecting cellular iron export is GAPDH. A specific post translationally modified isoform of GAPDH is recruited to the surface of iron loaded cells where it recruits apo-transferrin in close proximity to ferroportin so as to rapidly chelate the iron extruded.
The expression of hepcidin, which only occurs in certain cell types such as hepatocyte
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, ...
s, is tightly controlled at the transcriptional level and it represents the link between cellular and systemic iron homeostasis due to hepcidin's role as "gatekeeper" of iron release from enterocytes into the rest of the body. Erythroblasts
A nucleated red blood cell (NRBC), also known by several other names, is a red blood cell that contains a cell nucleus. Almost all vertebrate organisms have hemoglobin-containing cells in their blood, and with the exception of mammals, all of t ...
produce erythroferrone
Erythroferrone is a protein hormone encoded in humans by the ''ERFE'' gene. Erythroferrone is produced by erythroblasts, inhibits the production of hepcidin in the liver, and so increases the amount of iron available for hemoglobin synthesis. Ske ...
, a hormone which inhibits hepcidin and so increases the availability of iron needed for hemoglobin synthesis.
=Translational control of cellular iron
= Although some control exists at the transcriptional level, the regulation of cellular iron levels is ultimately controlled at the translational level byiron-responsive element-binding protein
The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR
, bind to iron-responsive elements (IREs) in the regulation of human iron metabolism.
Function
ACO1, or IRP1, is a bifunctional protein that functions as an ...
s IRP1 and especially IRP2. When iron levels are low, these proteins are able to bind to iron-responsive element
In molecular biology, the iron response element or iron-responsive element (IRE) is a short conserved stem-loop which is bound by iron response proteins (IRPs, also named IRE-BP or IRBP). The IRE is found in UTRs (untranslated regions) of vari ...
s (IREs). IREs are stem loop structures in the untranslated regions (UTRs) of mRNA.
Both ferritin and ferroportin contain an IRE in their 5' UTRs, so that under iron deficiency their translation is repressed by IRP2, preventing the unnecessary synthesis of storage protein and the detrimental export of iron. In contrast, TFR1 and some DMT1 variants contain 3' UTR IREs, which bind IRP2 under iron deficiency, stabilizing the mRNA, which guarantees the synthesis of iron importers.
Marine systems
Iron plays an essential role in marine systems and can act as a limiting nutrient for planktonic activity. Because of this, too much of a decrease in iron may lead to a decrease in growth rates in phytoplanktonic organisms such as diatoms. Iron can also be oxidized by marine microbes under conditions that are high in iron and low in oxygen. Iron can enter marine systems through adjoining rivers and directly from the atmosphere. Once iron enters the ocean, it can be distributed throughout the water column through ocean mixing and through recycling on the cellular level. In the arctic, sea ice plays a major role in the store and distribution of iron in the ocean, depleting oceanic iron as it freezes in the winter and releasing it back into the water when thawing occurs in the summer. The iron cycle can fluctuate the forms of iron from aqueous to particle forms altering the availability of iron to primary producers. Increased light and warmth increases the amount of iron that is in forms that are usable by primary producers.References
{{DEFAULTSORT:Iron In Biology Biological systems Biology and pharmacology of chemical elements Dietary mineralsBiology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
Nutrition
Physiology