Ionic liquids in carbon capture on:
[Wikipedia]
[Google]
[Amazon]
The use of ionic liquids in carbon capture is a potential application of
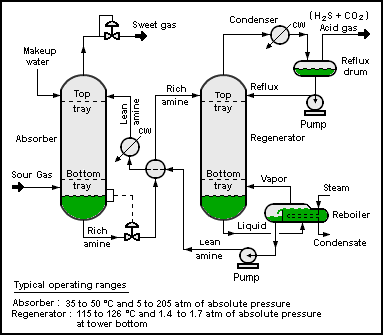 A typical CO2 absorption process consists of a feed gas, an absorption column, a stripper column, and output streams of CO2-rich gas to be sequestered, and CO2-poor gas to be released to the atmosphere. Ionic liquids could follow a similar process to
A typical CO2 absorption process consists of a feed gas, an absorption column, a stripper column, and output streams of CO2-rich gas to be sequestered, and CO2-poor gas to be released to the atmosphere. Ionic liquids could follow a similar process to
 As required for all separation techniques, ionic liquids exhibit selectivity towards one or more of the phases of a mixture.
As required for all separation techniques, ionic liquids exhibit selectivity towards one or more of the phases of a mixture.
ionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of ...
s as absorbents for use in carbon capture Carbon capture may refer to:
* Carbon capture and utilization, where the captured carbon dioxide is used
* Carbon sequestration, where the captured carbon dioxide is stored
** Carbon capture and storage, referring to carbon sequestration from point ...
and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile materials that have been considered for many applications. The urgency of climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
has spurred research into their use in energy-related applications such as carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
.
Carbon capture using absorption
Ionic liquids as solvents
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s are the most prevalent absorbent in postcombustion carbon capture technology today. In particular, monoethanolamine
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid wit ...
(MEA) has been used in industrial scales in postcombustion carbon capture, as well as in other CO2 separations, such as "sweetening" of natural gas. However, amines are corrosive, degrade over time, and require large industrial facilities. Ionic liquids on the other hand, have low vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
s . This property results from their strong Coulombic attractive force. Vapor pressure remains low through the substance's thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
point (typically >300 °C). In principle, this low vapor pressure simplifies their use and makes them "green
Green is the color between cyan and yellow on the visible spectrum. It is evoked by light which has a dominant wavelength of roughly 495570 Nanometre, nm. In subtractive color systems, used in painting and color printing, it is created by ...
" alternatives. Additionally, it reduces risk of contamination of the CO2 gas stream and of leakage into the environment.
The solubility of CO2 in ionic liquids is governed primarily by the anion, less so by the cation. The hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this anio ...
(PF6–) and tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchl ...
(BF4–) anions have been shown to be especially amenable to CO2 capture.
Ionic liquids have been considered as solvents in a variety of liquid-liquid extraction processes, but never commercialized. Beside that, ionic liquids have replaced the conventional volatile solvents in industry such as absorption of gases or extractive distillation. Additionally, ionic liquids are used as co-solutes for the generation of aqueous biphasic
Biphasic, meaning having two phases, may refer to:
* Phase (matter), in the physical sciences, a biphasic system, e.g. one involving liquid water and steam
* Biphasic sleep, a nap or siesta in addition to the usual sleep episode at night
* Phase ...
systems, or purification of biomolecules.
Process
 A typical CO2 absorption process consists of a feed gas, an absorption column, a stripper column, and output streams of CO2-rich gas to be sequestered, and CO2-poor gas to be released to the atmosphere. Ionic liquids could follow a similar process to
A typical CO2 absorption process consists of a feed gas, an absorption column, a stripper column, and output streams of CO2-rich gas to be sequestered, and CO2-poor gas to be released to the atmosphere. Ionic liquids could follow a similar process to amine gas treating
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and c ...
, where the CO2 is regenerated in the stripper using higher temperature. However, ionic liquids can also be stripped using pressure swings or inert gases, reducing the process energy requirement. A current issue with ionic liquids for carbon capture is that they have a lower working capacity than amines. Task-specific ionic liquids that employ chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
and physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals force. Even ...
are being developed in an attempt to increase the working capacity. 1-butyl-3-propylamineimidazolium tetrafluoroborate is one example of a TSIL.
Drawbacks
Selectivity
In carbon capture an effective absorbent is one which demonstrates a high selectivity, meaning that CO2 will preferentially dissolve in the absorbent compared to other gaseous components. In post-combustion carbon capture the most salient separation is CO2 from N2, whereas in pre-combustion separation CO is primarily separated from H2. Other components and impurities may be present in theflue gas
Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced a ...
, such as hydrocarbons, SO2, or H2S. Before selecting the appropriate solvent to use for carbon capture it is critical to ensure that at the given process conditions and flue gas composition CO2 maintains a much higher solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
in the solvent than the other species in the flue gas and thus has a high selectivity.
The selectivity of CO2 in ionic liquids has been widely studied by researchers. Generally, polar molecules
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
and molecules with an electric quadrupole moment are highly soluble in liquid ionic substances. It has been found that at high process temperatures the solubility of CO2 decreases, while the solubility of other species, such as CH4 and H2, may increase with increasing temperature, thereby reducing the effectiveness of the solvent. However, the solubility of N2 in ionic liquids is relatively low and does not increase with increasing temperature so the use of ionic liquids in post-combustion carbon capture may be appropriate due to the consistently high CO2/N2 selectivity. The presence of common flue gas impurities such as H2S severely inhibits CO2 solubility in ionic liquids and should be carefully considered by engineers when choosing an appropriate solvent for a particular flue gas.
Viscosity
A primary concern with the use of ionic liquids for carbon capture is their highviscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
compared with that of commercial solvents. Ionic liquids which employ chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
depend on a chemical reaction between solute and solvent for CO2 separation. The rate of this reaction is dependent on the diffusivity of CO2 in the solvent and is thus inversely proportional to viscosity. The self diffusivity of CO2 in ionic liquids are generally to the order of 10−10 m2/s, approximately an order of magnitude less than similarly performing commercial solvents used on CO2 capture. The viscosity of an ionic liquid can vary significantly according to the type of anion and cation, the alkyl chain length, and the amount of water or other impurities in the solvent. Because these solvents can be “designed” and these properties chosen, developing ionic liquids with lowered viscosities is a current topic of research. Supported ionic liquid phases (SILPs) are one proposed solution to this problem.
Tunability
 As required for all separation techniques, ionic liquids exhibit selectivity towards one or more of the phases of a mixture.
As required for all separation techniques, ionic liquids exhibit selectivity towards one or more of the phases of a mixture. 1-Butyl-3-methylimidazolium hexafluorophosphate
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMI ...
(BMIM-PF6) is a room-temperature ionic liquid that was identified early on as a viable substitute for volatile organic solvents in liquid-liquid separations. Other F6 and F4 containing ionic liquids have been studied for their CO2 absorption properties, as well as 1-ethyl-3-methylimidazolium (EMIM) and unconventional cations like trihexyl(tetradecyl) phosphonium ( 66614. Selection of different anion and cation combinations in ionic liquids affects their selectivity and physical properties. Additionally, the organic cations in ionic liquids can be "tuned" by changing chain lengths or by substituting radicals. Finally, ionic liquids can be mixed with other ionic liquids, water, or amines to achieve different properties in terms of absorption capacity and heat of absorption. This tunability has led some to call ionic liquids "designer solvents." 1-butyl-3-propylamineimidazolium tetrafluoroborate was specifically developed for CO2 capture; it is designed to employ chemisorption to absorb CO2 and maintain efficiency under repeated absorption/regeneration cycles. Other ionic liquids have been simulated or experimentally tested for potential use as CO2 absorbents.
Proposed industrial applications
Currently, CO2 capture uses mostlyamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
-based absorption technologies, which are energy intensive and solvent intensive. Volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
s alone in chemical processes represent a multibillion-dollar industry. Therefore, ionic liquids offer an alternative that prove attractive should their other deficiencies be addressed.
During the capture process, the anion and cation play a crucial role in the dissolution of CO2. Spectroscopic results suggest a favorable interaction between the anion and CO2, wherein CO2 molecules preferentially attach to the anion. Furthermore, intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s, such as hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, van der Waals bond
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and the ...
s, and electrostatic
Electrostatics is a branch of physics that studies electric charges at rest (static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber ...
attraction, contributes to the solubility of CO2 in ionic liquids. This makes ionic liquids promising candidates for CO2 capture because the solubility of CO2 can be modeled accurately by the regular solubility theory (RST), which reduces operational costs in developing more sophisticated model to monitor the capture process.
References
Further reading
# # {{cite journal, last1=Camper, first1=Dean, last2=Bara, first2=Jason E., last3=Gin, first3=Douglas L., last4=Noble, first4=Richard D., title=Room-Temperature Ionic Liquid−Amine Solutions: Tunable Solvents for Efficient and Reversible Capture of CO2, journal=Industrial & Engineering Chemistry Research, volume=47, issue=21, year=2008, pages=8496–8498, issn=0888-5885, doi=10.1021/ie801002m Carbon capture and storage Ions Ionic liquids