hydroboration on:
[Wikipedia]
[Google]
[Amazon]
In
 If BH3 is used as the hydroborating reagent, reactions typically proceed beyond the monoalkyl borane compounds, especially for less sterically hindered small olefins. Trisubstituted olefins can rapidly produce dialkyl boranes, but further alkylation of the organoboranes is slowed because of steric hindrance. This significant rate difference in producing di- and tri-alkyl boranes is useful in the synthesis of bulky boranes that can enhance regioselectivity (see below).
If BH3 is used as the hydroborating reagent, reactions typically proceed beyond the monoalkyl borane compounds, especially for less sterically hindered small olefins. Trisubstituted olefins can rapidly produce dialkyl boranes, but further alkylation of the organoboranes is slowed because of steric hindrance. This significant rate difference in producing di- and tri-alkyl boranes is useful in the synthesis of bulky boranes that can enhance regioselectivity (see below).
 Hydroboration of 1,2-disubstituted alkenes, such as a ''cis'' or ''trans'' olefin, produces generally a mixture of the two organoboranes of comparable amounts, even if the substituents are very different in terms of steric bulk. For such 1,2-disubstituted olefins, regioselectivity can be observed only when one of the two substituents is a phenyl ring. In such cases, such as ''trans''-1-phenylpropene, the boron atom is placed on the carbon adjacent to the phenyl ring. The observations above indicate that the addition of H-B bond to olefins is under electronic control rather than steric control.
Hydroboration of 1,2-disubstituted alkenes, such as a ''cis'' or ''trans'' olefin, produces generally a mixture of the two organoboranes of comparable amounts, even if the substituents are very different in terms of steric bulk. For such 1,2-disubstituted olefins, regioselectivity can be observed only when one of the two substituents is a phenyl ring. In such cases, such as ''trans''-1-phenylpropene, the boron atom is placed on the carbon adjacent to the phenyl ring. The observations above indicate that the addition of H-B bond to olefins is under electronic control rather than steric control.

 Hydroboration can also lead to amines by treating the intermediate organoboranes with
Hydroboration can also lead to amines by treating the intermediate organoboranes with

 Monoalkyl boranes are relatively rare. When the alkyl group is small, such as methyl, the monoalkylboranes tend to redistribute to give mixtures of diborane and di- and trialkylboranes. Monoalkylboranes typically exist as dimers of the form BH2sub>2. One example is thexylborane (ThxBH2), produced by the hydroboration of tetramethylethylene:
:B2H6 + 2 Me2C=CMe2 → e2CHCMe2BH2sub>2
A chiral example is monoisopinocampheylborane. Although often written as IpcBH2, it is a dimer pcBH2sub>2. It is obtained by hydroboration of (−)‐α‐pinene with
Monoalkyl boranes are relatively rare. When the alkyl group is small, such as methyl, the monoalkylboranes tend to redistribute to give mixtures of diborane and di- and trialkylboranes. Monoalkylboranes typically exist as dimers of the form BH2sub>2. One example is thexylborane (ThxBH2), produced by the hydroboration of tetramethylethylene:
:B2H6 + 2 Me2C=CMe2 → e2CHCMe2BH2sub>2
A chiral example is monoisopinocampheylborane. Although often written as IpcBH2, it is a dimer pcBH2sub>2. It is obtained by hydroboration of (−)‐α‐pinene with
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, hydroboration refers to the addition of a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
-boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
bond to certain double
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* ...
and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s involving carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
(, , , and ). This chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
is useful in the organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
of organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s.
Hydroboration produces organoborane compound
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
Organoboron ...
s that react with a variety of reagents to produce useful compounds, such as alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
s, or alkyl halides. The most widely known reaction of the organoboranes is oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
to produce alcohols typically by hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
. This type of reaction has promoted research on hydroboration because of its mild condition and a wide scope of tolerated alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
s. Another research subtheme is metal-catalysed hydroboration In chemistry, metal-catalysed hydroboration is a reaction used in organic synthesis. It is one of several examples of homogeneous catalysis.
History
In 1975, Kono and Ito reported that Wilkinson's catalyst (Rh(PPh3)3Cl) can undergo oxidative addit ...
.
The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
to Herbert C. Brown
Herbert Charles Brown (May 22, 1912 – December 19, 2004) was an American chemist and recipient of the 1979 Nobel Prize in Chemistry for his work with organoboranes.
Life and career
Brown was born Herbert Brovarnik in London, to Ukrainian Jewis ...
. He shared the prize with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates.
Addition of a H-B bond to C-C double bonds
Hydroboration is typically anti-Markovnikov, i.e. the hydrogen adds to the most substituted carbon of the double bond. That the regiochemistry is reverse of a typical HX addition reflects the polarity of the Bδ+-Hδ− bonds. Hydroboration proceeds via a four-membered transition state: the hydrogen and the boron atoms added on the same face of the double bond. Granted that the mechanism is concerted, the formation of the C-B bond proceeds slightly faster than the formation of the C-H bond. As a result, in the transition state, boron develops a partially negative charge while the more substituted carbon bears a partially positive charge. This partial positive charge is better supported by the more substituted carbon. Formally, the reaction is an example of agroup transfer reaction
In organic chemistry, a group transfer reaction is a pericyclic process where one or more groups of atoms is transferred from one molecule to another. They can sometimes be difficult to identify when separate reactant molecules combine into a sing ...
. However, an analysis of the orbitals involved reveals that the reaction is 'pseudopericyclic' and not subject to the Woodward–Hoffmann rules for pericyclic reactivity.
 If BH3 is used as the hydroborating reagent, reactions typically proceed beyond the monoalkyl borane compounds, especially for less sterically hindered small olefins. Trisubstituted olefins can rapidly produce dialkyl boranes, but further alkylation of the organoboranes is slowed because of steric hindrance. This significant rate difference in producing di- and tri-alkyl boranes is useful in the synthesis of bulky boranes that can enhance regioselectivity (see below).
If BH3 is used as the hydroborating reagent, reactions typically proceed beyond the monoalkyl borane compounds, especially for less sterically hindered small olefins. Trisubstituted olefins can rapidly produce dialkyl boranes, but further alkylation of the organoboranes is slowed because of steric hindrance. This significant rate difference in producing di- and tri-alkyl boranes is useful in the synthesis of bulky boranes that can enhance regioselectivity (see below).
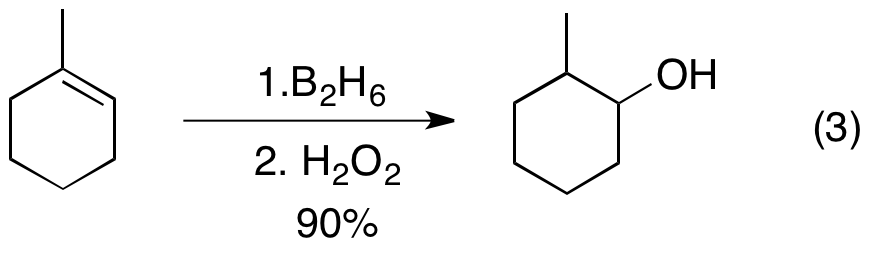
Reactions involving substituted alkenes
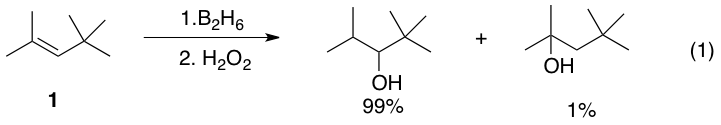
For trisubstitutedalkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
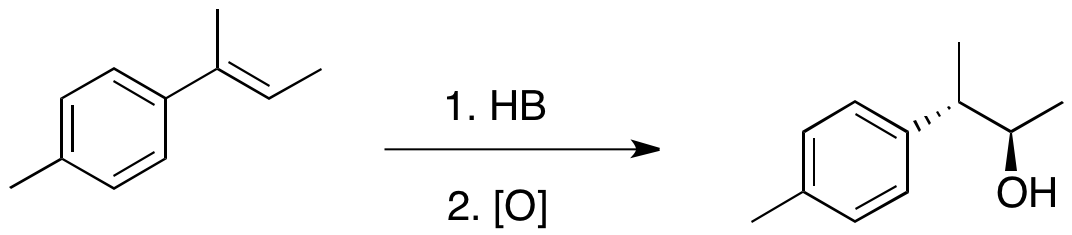
s such as 1, boron is predominantly placed on the less substituted carbon. The minor product, in which the boron atom is placed on the more substituted carbon, is usually produced in less than 10%. A notable case with lower regioselectivity is styrene, and the selectivity is strongly influenced by the substituent on the para position.
 Hydroboration of 1,2-disubstituted alkenes, such as a ''cis'' or ''trans'' olefin, produces generally a mixture of the two organoboranes of comparable amounts, even if the substituents are very different in terms of steric bulk. For such 1,2-disubstituted olefins, regioselectivity can be observed only when one of the two substituents is a phenyl ring. In such cases, such as ''trans''-1-phenylpropene, the boron atom is placed on the carbon adjacent to the phenyl ring. The observations above indicate that the addition of H-B bond to olefins is under electronic control rather than steric control.
Hydroboration of 1,2-disubstituted alkenes, such as a ''cis'' or ''trans'' olefin, produces generally a mixture of the two organoboranes of comparable amounts, even if the substituents are very different in terms of steric bulk. For such 1,2-disubstituted olefins, regioselectivity can be observed only when one of the two substituents is a phenyl ring. In such cases, such as ''trans''-1-phenylpropene, the boron atom is placed on the carbon adjacent to the phenyl ring. The observations above indicate that the addition of H-B bond to olefins is under electronic control rather than steric control.


Reactions of organoboranes
The C-B bonds generated by hydroboration are reactive with various reagents, the most common one beinghydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
. Because the addition of H-B to olefins is stereospecific, this oxidation reaction will be diastereoselective when the alkene is trisubstituted. Hydroboration-oxidation is thus an excellent way of producing alcohols in a stereospecific and anti-Markovnikov fashion.
 Hydroboration can also lead to amines by treating the intermediate organoboranes with
Hydroboration can also lead to amines by treating the intermediate organoboranes with monochloramine
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting p ...
or O-hydroxylaminesulfonic acid (HSA).
Terminal olefins are converted to the corresponding alkyl bromide
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
One prominent application of synthetic organobromine compounds is the ...
s and alkyl iodides by treating the organoborane intermediates with bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
or iodine. Such reactions have not however proven very popular, because succinimide based reagents such as NIS and NBS are more versatile and do not require rigorous conditions as do organoboranes.
etc.
Borane adducts
160px, Borane dimethylsulfide (BMS) is a complexed borane reagent that is widely used for hydroborations. Diborane can be producedin situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...
by reduction BF3 with NaBH4 (see for Flavopiridol). Usually however, borane dimethylsulfide complex BH3S(CH3)2 (BMS) is used as a source of BH3. It can be obtained in highly concentrated forms.
The adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
BH3(THF) is also commercially available as THF solutions wherein it exists as the 1:1 adduct. It degrades with time.
Borane adducts with phosphines and amines are also available, but are not widely used. Borane makes a strong adduct with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
; using this adduct requires harsher conditions in hydroboration. This can be advantageous for cases such as hydroborating trienes to avoid polymerization. More sterically hindered tertiary and silyl amines can deliver borane to alkenes at room temperature.

Monosubstituted boranes
borane dimethyl sulfide
Borane dimethylsulfide (BMS) is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS ...
.
Species of the form RBH2 are available for R = alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
and halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a flu ...
. Monobromo- and monochloro-borane can be prepared from BMS and the corresponding boron trihalides. The stable complex of monochloroborane and 1,4-dioxane effects hydroboration of terminal alkenes.
Disubstituted boranes
Dimesitylborane
dimesitylborane is a dimer (C6H2Me3)2B2H2). It reacts only slowly with simple terminal alkenes. On the other hand, alkynes undergo monohydroboration with Mes2BH easily to produce alkenylboranes.Disiamylborane
Among hindered dialkylboranes is disiamylborane, abbreviated Sia2BH. It also is a dimer. Owing to its steric bulk, it selectively hydroborates less hindered, usually terminal alkenes in the presence of more substituted alkenes. Disiamylborane must be freshly prepared as its solutions can only be stored at 0 °C for a few hours. Dicyclohexylborane Chx2BH exhibits improved thermal stability than Sia2BH.9-BBN
A versatile dialkylborane is 9-BBN. Also called "banana borane", it exists as a dimer. It can be distilled without decomposition at 195 °C (12mm Hg). Reactions with 9-BBN typically occur at 60–80 °C, with most alkenes reacting within one hour. Tetrasubstituted alkenes add 9-BBN at elevated temperature. Hydroboration of alkenes with 9-BBN proceeds with excellent regioselectivity. It is more sensitive to steric differences than Sia2BH, perhaps because of it rigid C8 backbone. 9-BBN is more reactive towards alkenes than alkynes.Other secondary boranes
Simple, unhindered dialkylboranes are reactive at room temperature towards most alkenes and terminal alkynes but are difficult to prepare in high purity, since they exist in equilibrium with mono- and trialkylboranes. One common way of preparing them is the reduction of dialkylhalogenoboranes with metal hydrides. An important synthetic application using such dialkylboranes, such as diethylborane, is the transmetallation of the organoboron compounds to form organozinc compounds.Pinacolborane and catecholborane
For catalytic hydroboration, pinacolborane andcatecholborane
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.
Synthesis and structure
Traditionally catecholborane ...
are widely used. They also exhibit higher reactivity toward alkynes. Pinacolborane is also widely used in a catalyst-free hydroborations.
See also
* Hydroboration–oxidation reactionReferences
{{Alkenes Organic reactions