Haloalkene on:
[Wikipedia]
[Google]
[Amazon]
In
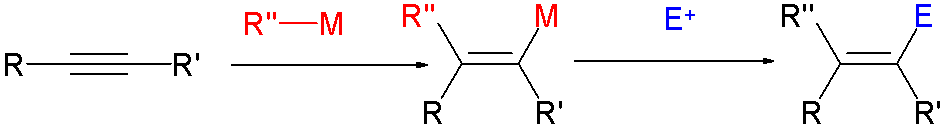 *
*  * Stork-Zhao olefination - a modification of the
* Stork-Zhao olefination - a modification of the  *
*
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, a vinyl halide is a compound with the formula CH2=CHX (X = halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
). The term vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl halides. From the perspective of applications, the dominant member of this class of compounds is vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, which is produced on the scale of millions of tons per year as a precursor to polyvinyl chloride. Polyvinyl fluoride
Polyvinyl fluoride (PVF) or –(CH2CHF)n– is a polymer material mainly used in the flammability-lowering coatings of airplane interiors and photovoltaic module backsheets. It is also used in raincoats and metal sheeting. Polyvinyl fluoride is a ...
is another commercial product. Related compounds include vinylidene chloride
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula CHCl. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, ...
and vinylidene fluoride
1,1-Difluoroethylene, also known as vinylidene fluoride, is a hydrofluoroolefin. It is a flammable gas. Global production in 1999 was approximately 33,000 metric tons. It is primarily used in the production of fluoropolymers such as polyvinyliden ...
.
Synthesis
Vinyl chloride is produced by dehydrochlorination of 1,2-dichloroethane. Due to their high utility, many approaches to vinyl halides have been developed, such as: * reactions of vinylorganometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
species with halogens
 *
* Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, referencing Kazuhiko Takai, who first reported it in 1986. In the original reaction, ...
Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
 *
* Olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
Reactions
Vinyl bromide and related alkenyl halides form theGrignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
and related organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s. Alkenyl halides undergo base elimination to give the corresponding alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. Most important is their use in cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s (e.g. Suzuki-Miyaura coupling
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
, Stille coupling
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electroph ...
, Heck coupling
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
, etc.).
See also
* Vinyl iodide functional groupReferences
{{Organohalide-stub Organohalides