Haldane effect on:
[Wikipedia]
[Google]
[Amazon]
The Haldane effect is a property of
 By
By
hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
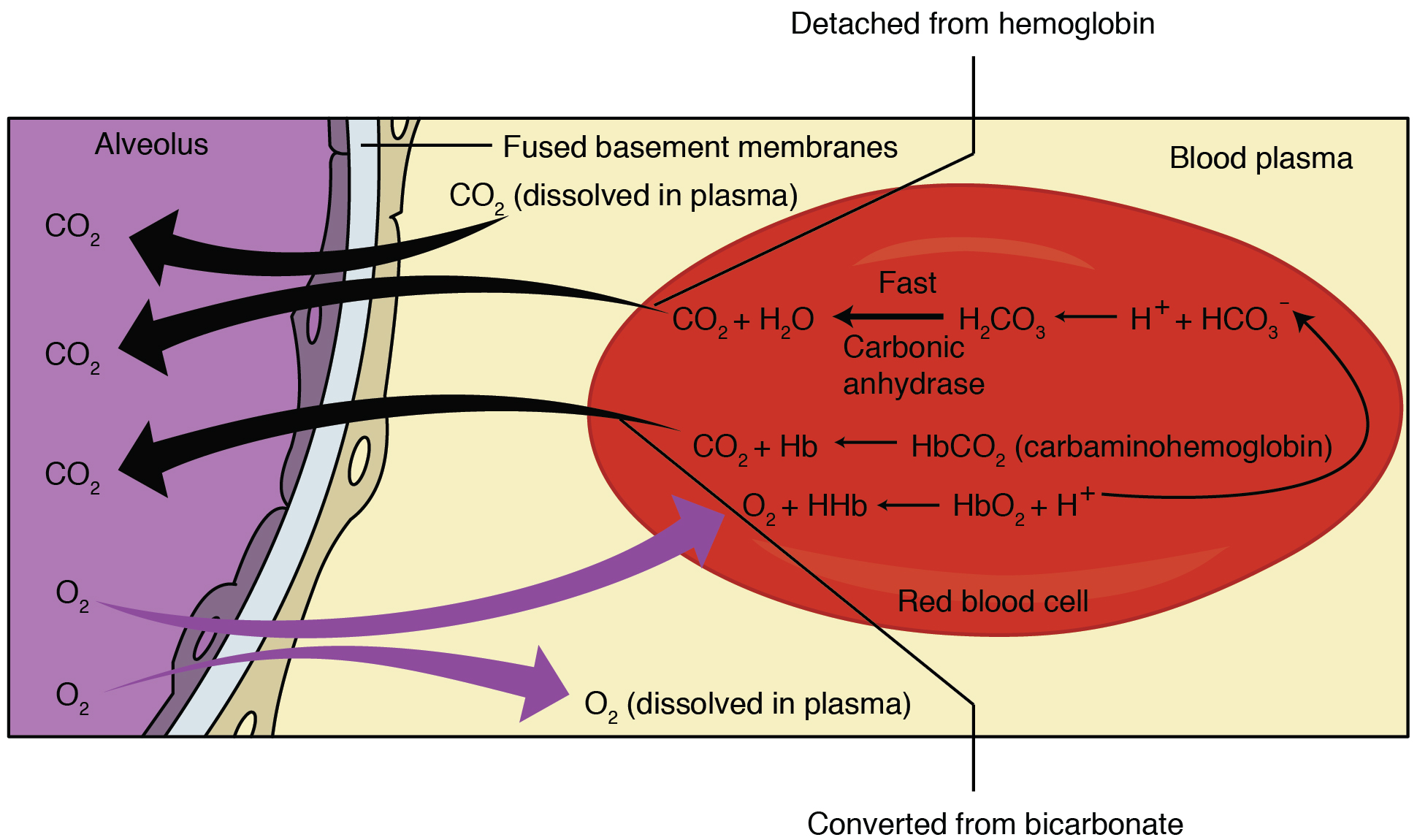
first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. A high concentration of CO2 facilitates dissociation of oxyhemoglobin.
Carbaminohemoglobin
Carbon dioxide travels through theblood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
in three different ways. One of these ways is by binding to amino
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
groups, creating carbamino compounds. Amino groups are available for binding at the N-terminals and at side-chains of arginine and lysine residues in hemoglobin. When carbon dioxide binds to these residues carbaminohemoglobin
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. Twenty-three percent of ...
is formed. This amount of carbaminohemoglobin
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. Twenty-three percent of ...
formed is inversely proportional to the amount of oxygen attached to hemoglobin. Thus, at lower oxygen saturation, more carbaminohemoglobin
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. Twenty-three percent of ...
is formed. These dynamics explain the relative difference in hemoglobin's affinity for carbon dioxide depending on oxygen levels known as the Haldane effect.
Buffering
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
residues in hemoglobin can accept and act as buffers. Deoxygenated hemoglobin is a better proton acceptor than the oxygenated form.
In red blood cells, the enzyme carbonic anhydrase catalyzes the conversion of dissolved carbon dioxide to carbonic acid, which rapidly dissociates to bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochem ...
and a free proton:
:CO2 + H2O → H2CO3 → H+ + HCO3− By
By Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
, anything that stabilizes the proton produced will cause the reaction to shift to the right, thus the enhanced affinity of deoxyhemoglobin for protons enhances synthesis of bicarbonate and accordingly increases capacity of deoxygenated blood for carbon dioxide. The majority of carbon dioxide in the blood is in the form of bicarbonate. Only a very small amount is actually dissolved as carbon dioxide, and the remaining amount of carbon dioxide is bound to hemoglobin.
In addition to enhancing removal of carbon dioxide from oxygen-consuming tissues, the Haldane effect promotes dissociation of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
from hemoglobin in the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
. In the oxygen-rich capillaries of the lung, this property causes the displacement of carbon dioxide to plasma as low-oxygen blood enters the alveolus Alveolus (; pl. alveoli, adj. alveolar) is a general anatomical term for a concave cavity or pit.
Uses in anatomy and zoology
* Pulmonary alveolus, an air sac in the lungs
** Alveolar cell or pneumocyte
** Alveolar duct
** Alveolar macrophage
* Mam ...
and is vital for alveolar gas exchange.
The general equation for the Haldane Effect is:
:H+ + HbO2 H+Hb + O2;
However, this equation is confusing as it reflects primarily the Bohr effect. The significance of this equation lies in realizing that oxygenation of Hb promotes dissociation of H+ from Hb, which shifts the bicarbonate buffer equilibrium towards CO2 formation; therefore, CO2 is released from RBCs.
Clinical significance
In patients with lung disease, lungs may not be able to increase alveolar ventilation in the face of increased amounts of dissolved CO2. This partially explains the observation that some patients with emphysema might have an increase in PaCO2 (partial pressure of arterial dissolved carbon dioxide) following administration of supplemental oxygen even if content of CO2 stays equal.See also
* Bohr effect * Chloride shiftReferences
External links
* {{Respiratory physiology Hematology Hemoproteins Respiratory physiology