Hydrosilane on:
[Wikipedia]
[Google]
[Amazon]
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is

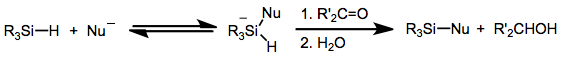
 The reaction is stoichiometric.
The reaction is stoichiometric.
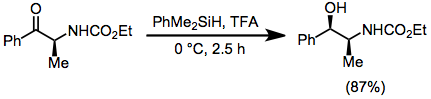
silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Sila ...
(SiH4). Commonly, hydrosilane refers to organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic co ...
derivatives. Examples include phenylsilane
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6 H5 SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have ...
(PhSiH3) and triethoxysilane ((C2H5O)3SiH). Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks.
Synthesis
Trichlorosilane
Trichlorosilane is an inorganic compound with the formula HCl3Si. It is a colourless, volatile liquid. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry. In water, it rapidly decomposes to pr ...
is produced commercially by the reaction of hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
with silicon:
:Si + 3 HCl → HSiCl3 + H2
Many alkoxy hydrosilanes are generated by alcoholysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
of trichlorosilane. One example is triethoxysilane:
:HSiCl3 + 3EtOH → HSi(OEt)3 + 3 HCl
Organohydrosilanes can be prepared by partial hydrosilation Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009 ...
of silane itself:
:SiH4 + 3 C2H4 → HSi(C2H5)3
In the laboratory, hydrosilanes classically are prepared by treating chlorosilanes with hydride reagents, such as lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
:
:4ClSi(C2H5)3 + LiAlH4 → 4HSi(C2H5)3 + LiAlCl4
Structure
The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm). The Si-H bond is about 10% weaker compared to C-H bonds. Hydrogen is moreelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than silicon (hence the naming convention of silyl hydrides), which results in the polarization of the Si-H bond to be the reverse of that for the C-H bond. Generally silyl hydrides are colourless with physical properties (solubility, volatility) comparable to hydrocarbons. They can be pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
, reflecting the great driving force for replacing Si-H bonds with Si-O bonds.
Reactions and applications
Setting aside silane itself, for which is used mainly in the microelectronics industry as a source of Si, hydrosilanes participate in many reactions. Hydrosilanes are mainly used for diverse styles of reduction in both industrial and laboratory-scale reactions. These including deoxygenation, hydrosilylation, andionic hydrogenation Ionic hydrogenation refers to hydrogenation achieved by the addition of a hydride to substrate that has been activated by an electrophile. Some ionic hydrogenations entail addition of H2 to the substrate and some entail replacement of a heteroatom ...
. Hydrosilylation
SInhydrosilylation Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009 ...
, the Si-H bond adds across multiple bonds in alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s, and carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s. The reaction of alkenes is commercially significant. Many organosilicon compounds and materials are prepared in this way. Illustrative is the crosslinking of vinyl-terminated siloxanes:

Conversion to silanols
In the presence of platinum-based catalysts, hydrosilanes react with water to give silanols: :R3SiH + H2O → R3SiOH + H2 The same transformation can be effected with oxygen in the presence of catalysts.Fluoride complexes
In the presence of fluoride ions, hydrosilanes reversibly form hypervalent fluorosilicates with the formula R3Si(F)H−). These species are reducing agents, akin toborohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihyd ...
.
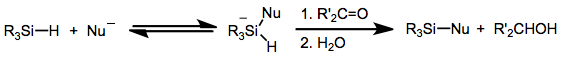
Ionic hydrogenation
Reductions with hydrosilanes are a subset ofionic hydrogenation Ionic hydrogenation refers to hydrogenation achieved by the addition of a hydride to substrate that has been activated by an electrophile. Some ionic hydrogenations entail addition of H2 to the substrate and some entail replacement of a heteroatom ...
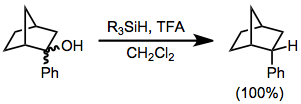
s. In this type of reaction, carbocations are generated by the action of strong Lewis or Brønsted acids in the presence of hydrosilanes, which then transfer hydride. A typical acid is trifluoroacetic acid (TFA).
: The reaction is stoichiometric.
The reaction is stoichiometric.
Deoxygenation and ionic hydrogenation
Hydrosilanes are used for thedeoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
of phosphine oxides and sulfoxides.
Hydrosilanes serve as hydride donors in some ionic hydrogenations Ionic hydrogenation refers to hydrogenation achieved by the addition of a hydride to substrate that has been activated by an electrophile. Some ionic hydrogenations entail addition of H2 to the substrate and some entail replacement of a heteroatom w ...
.
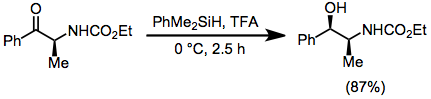
Coordination the metals
Hydrosilanes form sigma complexes with unsaturated metals. The bonding is similar to that indihydrogen complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3( PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions i ...
es but stronger. One example is (CH3C5H4)Mn(CO)2(H2SiPh2). Such adducts represent models for and competitors with the oxidative addition of the Si-H bond.
Reduction of or addition to organic substrates
Akin to the hydrosilylation of alkenes, hydrosilanes add to a variety of unsaturated substrates. In one example, PMHS. In one studytriethylsilane
Triethylsilane is the organosilicon compound with the formula (C2H5)3SiH. It is a trialkylsilane. The Si-H bond is reactive. This colorless liquid is used in organic synthesis as a reducing agent and as a precursor to silyl ethers. As one of the ...
is used in the conversion of a phenyl azide
Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear organic azide, azide substituent bound to a phenyl ...
to an aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
:
:
In this reaction ACCN is a radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical ini ...
and an aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
transfers radical character to the silylhydride. The triethylsilyl free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
:
:
Further reading
*Hydrogen-terminated silicon surface Hydrogen-terminated silicon surface is a chemically passivated silicon substrate where the surface Si atoms are bonded to hydrogen. The hydrogen-terminated surfaces are hydrophobic, luminescent, and amenable to chemical modification. Hydrogen-ter ...
Selective reading
*References
{{reflist Silanes