Hydrophosphination on:
[Wikipedia]
[Google]
[Amazon]
 Hydrophosphination is the insertion of a carbon-carbon multiple bond into a
Hydrophosphination is the insertion of a carbon-carbon multiple bond into a
 The reactions proceed by abstraction of an H atom the phosphine precursor, producing the phosphino radical, a seven electron species. This radical then adds to the alkene, and subsequent H-atom transfer completes the cycle. Some highly efficient hydrophosphinations appear not to proceed via radicals, but alternative explanations are lacking.
The reactions proceed by abstraction of an H atom the phosphine precursor, producing the phosphino radical, a seven electron species. This radical then adds to the alkene, and subsequent H-atom transfer completes the cycle. Some highly efficient hydrophosphinations appear not to proceed via radicals, but alternative explanations are lacking.
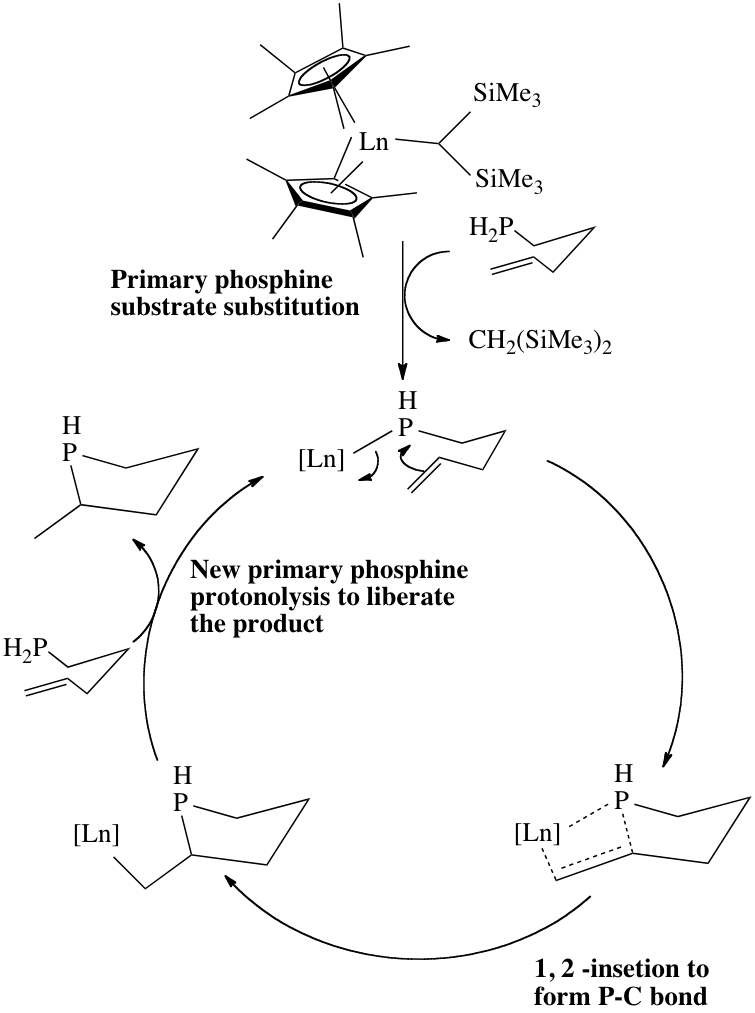 Metal complexes of ''d''0 configurations are effective catalysts for hydrophosphinations of simple alkenes and alkynes. Intramolecular reactions are facile, e.g. starting with ''α,ω''-pentenylphosphine. The primary phosphine undergoes a ''σ''-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex. Subsequently the pendant terminal alkene or alkyne inserts into the Ln-P bond. Finally, protonolysis of the Ln-C bond with the starting primary phosphine releases the new phosphine and regenerates the catalyst. Given that the metal is electron-poor, the M-C bond is sufficiently enough to be protonolyzed by the substrate primary phosphine.
Most metal catalyzed hydrophosphinations proceed via metal phosphido intermediates. Some however proceed by metal-phosphinidene intermediates, i.e. species with M=PR double bonds. One such example is the Ti-catalyzed hydrophosphination of diphenylacetylene with phenylphosphine. This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate. This precursor undergoes exchange with phenylphosphine to give the titanium-phenylphosphinidene complex, which is the catalyst. The Ti=PPh species undergoes a +2cycloaddition with diphenylacetylene to make the corresponding metallacyclobutene. The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Titanium-catalyzed 1,4-hydrophosphination of 1,3-dienes with
Metal complexes of ''d''0 configurations are effective catalysts for hydrophosphinations of simple alkenes and alkynes. Intramolecular reactions are facile, e.g. starting with ''α,ω''-pentenylphosphine. The primary phosphine undergoes a ''σ''-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex. Subsequently the pendant terminal alkene or alkyne inserts into the Ln-P bond. Finally, protonolysis of the Ln-C bond with the starting primary phosphine releases the new phosphine and regenerates the catalyst. Given that the metal is electron-poor, the M-C bond is sufficiently enough to be protonolyzed by the substrate primary phosphine.
Most metal catalyzed hydrophosphinations proceed via metal phosphido intermediates. Some however proceed by metal-phosphinidene intermediates, i.e. species with M=PR double bonds. One such example is the Ti-catalyzed hydrophosphination of diphenylacetylene with phenylphosphine. This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate. This precursor undergoes exchange with phenylphosphine to give the titanium-phenylphosphinidene complex, which is the catalyst. The Ti=PPh species undergoes a +2cycloaddition with diphenylacetylene to make the corresponding metallacyclobutene. The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Titanium-catalyzed 1,4-hydrophosphination of 1,3-dienes with

 Some late metal hydrophosphination catalysts proceed via
Some late metal hydrophosphination catalysts proceed via
 Hydrophosphination is the insertion of a carbon-carbon multiple bond into a
Hydrophosphination is the insertion of a carbon-carbon multiple bond into a phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
-hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
bond forming a new phosphorus-carbon bond. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is influenced by the catalyst. Catalysts take many forms, but most prevalent are bases and free-radical initiators.
Acid-base routes
The usual application of hydrophosphination involves reactions ofphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
(PH3). Typically base-catalysis allows addition of Michael acceptor
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
s such as acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular ...
to give tris(cyanoethyl)phosphine
Tris(cyanoethyl)phosphine is the organophosphorus compound with the formula P(CH2CH2CN)3. It is white solid that is air stable, which is unusual for a trialkylphosphine. It is prepared by the hydrophosphination of acrylonitrile with phosphine. ...
:
:PH3 + 3 CH2=CHZ → P(CH2CH2Z)3 (Z = NO2, CN, C(O)NH2)
Acid catalysis is applicable to hydrophosphination with alkenes that form stable carbocations. These alkenes include isobutylene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Productio ...
and many analogues:
:PH3 + R2C=CH2 → R2(CH3)CPH2 (R = Me, alkyl, etc)
Bases catalyze the addition of secondary phosphines to vinyldiphenylphosphine:
:HPR2 + CH2=CHPR'2 → R2PCH2CH2PR'2
Free-radical methods
Many hydrophosphination reactions are initiated byfree-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
s. AIBN and peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
s are typical initiators, as well as Ultraviolet irradiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
. In this way, the commercially important tributylphosphine
Tributylphosphine is the organophosphorus compound with the formula P(CH). Abbreviated or PBu, it is a tertiary phosphine. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with ...
and trioctylphosphine are prepared in good yields from 1-butene
1-Butene (or 1-Butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin. It is one of the isomers of butene (butylene). I ...
and 1-octene
1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thu ...
, respectively.
 The reactions proceed by abstraction of an H atom the phosphine precursor, producing the phosphino radical, a seven electron species. This radical then adds to the alkene, and subsequent H-atom transfer completes the cycle. Some highly efficient hydrophosphinations appear not to proceed via radicals, but alternative explanations are lacking.
The reactions proceed by abstraction of an H atom the phosphine precursor, producing the phosphino radical, a seven electron species. This radical then adds to the alkene, and subsequent H-atom transfer completes the cycle. Some highly efficient hydrophosphinations appear not to proceed via radicals, but alternative explanations are lacking.
Metal-catalyzed reactions
Metal-catalyzed hydrophosphinations are not widely used, although they have been extensively researched. Studies mainly focus on secondary and primary organophosphines (R2PH and RPH2, respectively). These substrates bind to metals, and the resulting adducts insert alkenes and alkynes into the P-H bonds via diverse mechanisms.Early transition metal and lanthanide catalysts
 Metal complexes of ''d''0 configurations are effective catalysts for hydrophosphinations of simple alkenes and alkynes. Intramolecular reactions are facile, e.g. starting with ''α,ω''-pentenylphosphine. The primary phosphine undergoes a ''σ''-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex. Subsequently the pendant terminal alkene or alkyne inserts into the Ln-P bond. Finally, protonolysis of the Ln-C bond with the starting primary phosphine releases the new phosphine and regenerates the catalyst. Given that the metal is electron-poor, the M-C bond is sufficiently enough to be protonolyzed by the substrate primary phosphine.
Most metal catalyzed hydrophosphinations proceed via metal phosphido intermediates. Some however proceed by metal-phosphinidene intermediates, i.e. species with M=PR double bonds. One such example is the Ti-catalyzed hydrophosphination of diphenylacetylene with phenylphosphine. This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate. This precursor undergoes exchange with phenylphosphine to give the titanium-phenylphosphinidene complex, which is the catalyst. The Ti=PPh species undergoes a +2cycloaddition with diphenylacetylene to make the corresponding metallacyclobutene. The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Titanium-catalyzed 1,4-hydrophosphination of 1,3-dienes with
Metal complexes of ''d''0 configurations are effective catalysts for hydrophosphinations of simple alkenes and alkynes. Intramolecular reactions are facile, e.g. starting with ''α,ω''-pentenylphosphine. The primary phosphine undergoes a ''σ''-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex. Subsequently the pendant terminal alkene or alkyne inserts into the Ln-P bond. Finally, protonolysis of the Ln-C bond with the starting primary phosphine releases the new phosphine and regenerates the catalyst. Given that the metal is electron-poor, the M-C bond is sufficiently enough to be protonolyzed by the substrate primary phosphine.
Most metal catalyzed hydrophosphinations proceed via metal phosphido intermediates. Some however proceed by metal-phosphinidene intermediates, i.e. species with M=PR double bonds. One such example is the Ti-catalyzed hydrophosphination of diphenylacetylene with phenylphosphine. This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate. This precursor undergoes exchange with phenylphosphine to give the titanium-phenylphosphinidene complex, which is the catalyst. The Ti=PPh species undergoes a +2cycloaddition with diphenylacetylene to make the corresponding metallacyclobutene. The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Titanium-catalyzed 1,4-hydrophosphination of 1,3-dienes with diphenylphosphine
Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.
Syn ...
has been demonstrated. It is a rare example of a ''d2'' catalyst. In the first step, the Ti(II) precursor inserted in the P-H bond of diphenylphosphine
Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.
Syn ...
(Ph2PH).
Late transition metal catalysts
Late transition metal hydrophosphination catalysts, i.e. those reliant on the nickel-triad and neighboring elements, generally require alkenes and alkynes with electron withdrawing substituents. A strong base is required as a cocatalyst.
 Some late metal hydrophosphination catalysts proceed via
Some late metal hydrophosphination catalysts proceed via oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
of a P-H bond. For example, a Pt(0) catalyst undergoes oxidative addition of a secondary phosphine to form the corresponding hydrido Pt(II) phosphido complex. These systems catalyze hydrophosphination of acrylonitrile, although this reaction can be achieved without metal catalysts. The key P-C bond-forming step occurs through an outer-sphere, Michael-type addition.
The usual mechanism for hydrophosphination for late metal catalysts involves insertion of the alkene into the metal-phosphorus bond. Insertion into the metal-hydrogen bond is also possible. The product phosphine is produced through reductive elimination of a P-C bond rather than a P-H bond in Glueck's system. The Ni(0) catalyst involves oxidation addition of a P-H bond to the metal, followed by insertion of the alkene into the M-H bond.Hydrophosphorylation and related reactions
Utilizing phosphorus(V) precursors hydrophosphorylation entails the insertion of alkenes and alkynes into the P-H bonds of secondary phosphine oxides: :R2P(O)H + CH2=CHR → R2P(O)CH2CH2R The reaction can be effected both using metal catalysts or free-radical initiators.Further reading
* * * * * * * * * * * * * * * * * * * *References
{{reflist, 30em Addition reactions Green chemistry Organophosphanes Stoichiometry