Hexadehydro Diels–Alder Reaction on:
[Wikipedia]
[Google]
[Amazon]
In
/ref>Holden, C.; Greaney, M. F. ''Angew. Chem. Int. Ed. Engl.'', 2014, ''53'', 574
/ref> This benzyne intermediate then reacts with a suitable trapping agent to form a substituted
/ref>Vandavasi, J. K.; Hu, W.-P.; Hsiao, C.-T.; Senadi, G. C.; Wang, J.-J. ''RSC Adv.'', 2014, ''4'', 5754
/ref> The prevailing mechanism for the thermally-initiated HDDA reaction is a +2cycloaddition between a conjugated diyne (1,3-dialkyne) and an alkyne (often referred to as a ''diynophile'' in analogy to the Diels–Alder dienophile) to form an ''ortho''- benzyne species. The metal-catalyzed HDDA is thought to proceed through a similar pathway, forming a metal-stabilized benzyne, which is then trapped. The simplest model of an HDDA reaction is the cycloaddition of butadiyne and
/ref> This reactive intermediate (denoted by brackets) subsequently reacts with a generalized trapping reagent that consists of a The o-benzyne intermediate can be visualized in the two
The o-benzyne intermediate can be visualized in the two
 The HDDA +2cycloaddition can occur via either a
The HDDA +2cycloaddition can occur via either a
/ref>

 Formally, the hexadehydro Diels–Alder reaction describes only the formation of the benzyne, but this species is an unstable intermediate that reacts readily with a variety of trapping partners, including reaction
Formally, the hexadehydro Diels–Alder reaction describes only the formation of the benzyne, but this species is an unstable intermediate that reacts readily with a variety of trapping partners, including reaction
/ref>Miyawaki, K.; Suzuki, R.; Kawano, T.; Ueda, I. ''Tetrahedron Lett.'', 1997, ''38'', 394
/ref> Johnson and co-workers observed the cyclization of 1,3,8-nonatriyne under pyrolysis, flash vacuum thermolysis (600 °C, 10−2 torr) to form two products,
/ref>

/ref>
/ref> The HDDA-generated benzyne can be trapped with a suitable ene donor that is covalently tethered to the benzyne. The benzyne serves as the enophile, while the ene can be an alkene (Alder ene) or an aromatic ring (aromatic ene). Lee and co-workers have shown an HDDA-Alder ene cascade reaction that can produce a variety of products, including medium-sized fused rings, spirocycles, and Hoye and co-workers demonstrated a thermally-initiated triple HDDA-aromatic ene-Alder ene cascade that leads to heavily functionalized products in one-step with no additional reagents or by-products.
Hoye and co-workers demonstrated a thermally-initiated triple HDDA-aromatic ene-Alder ene cascade that leads to heavily functionalized products in one-step with no additional reagents or by-products.

/ref> In the absence of external trapping reagents, the benzyne intermediate can abstract

/ref> The metal-complexed aryne intermediate can be trapped by the counterion to produce aryl rings with fluoro,


organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne (2 alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
functional groups arranged in a conjugated system
In physical organic chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases Chemical stability, stability. It is Reson ...
) and an alkyne to form a reactive benzyne species, via a +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reaction.Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. ''Nature'', 2012, ''490'', 20/ref>Holden, C.; Greaney, M. F. ''Angew. Chem. Int. Ed. Engl.'', 2014, ''53'', 574
/ref> This benzyne intermediate then reacts with a suitable trapping agent to form a substituted
aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
product. This reaction is a derivative of the established Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
and proceeds via a similar +2cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

Reaction mechanism
Depending on the substrate chosen, the HDDA reaction can be initiated thermally or by the addition of a suitablecatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, often a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
.Yun, S. Y.; Wang, K.-P.; Lee, N.-K.; Mamidipalli, P.; Lee, D. ''J. Am. Chem. Soc.'', 2013, ''135'', 466/ref>Vandavasi, J. K.; Hu, W.-P.; Hsiao, C.-T.; Senadi, G. C.; Wang, J.-J. ''RSC Adv.'', 2014, ''4'', 5754
/ref> The prevailing mechanism for the thermally-initiated HDDA reaction is a +2cycloaddition between a conjugated diyne (1,3-dialkyne) and an alkyne (often referred to as a ''diynophile'' in analogy to the Diels–Alder dienophile) to form an ''ortho''- benzyne species. The metal-catalyzed HDDA is thought to proceed through a similar pathway, forming a metal-stabilized benzyne, which is then trapped. The simplest model of an HDDA reaction is the cycloaddition of butadiyne and
acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
to form ortho-benzyne (o-benzyne, shown below).Ajaz, A.; Bradley, A. Z.; Burrell, R. C.; Li, W. H. H.; Daoust, K. J.; Bovee, L. B.; DiRico, K. J.; Johnson, R. P. ''J. Org. Chem.'', 2011, ''76'', 932/ref> This reactive intermediate (denoted by brackets) subsequently reacts with a generalized trapping reagent that consists of a
nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
(Nu-) and electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
(El-) site, giving the benzenoid product shown.
 The o-benzyne intermediate can be visualized in the two
The o-benzyne intermediate can be visualized in the two resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance struc ...
forms illustrated above. The most commonly depicted form is the alkyne (1), but the cumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumu ...
(1’) form can be helpful in visualizing ring formation by +2cycloaddition.
Thermodynamics and kinetics
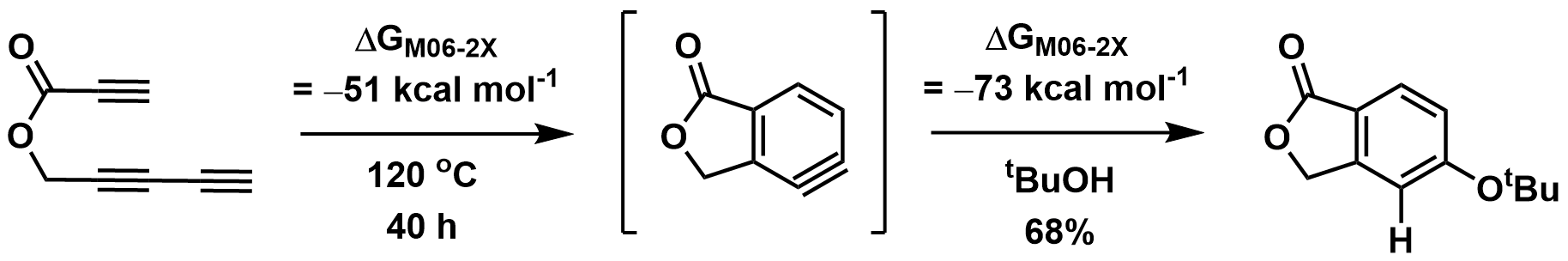
The HDDA reaction is often thermodynamically favorable (exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
), but can have a significant kinetic barrier to reaction (high activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
). Calculations have suggested that the formation of unsubstituted o-benzyne (from butadiyne and acetylene, above) has an activation energy of 36 kcal mol−1, but is thermodynamically favorable, estimated to be exothermic by -51 kcal mol−1. As a result of higher activation energy, some HDDA reactions require heating to elevated temperatures (>100 °C) in order to initiate.
Furthermore, the benzyne trapping step is also thermodynamically favourable, calculated to be an additional -73 kcal mol−1 for trapping of an ester-substituted o-benzyne with tert-butanol
''tert''-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as ''t''-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. ''tert''-Butyl alcohol is a colorless solid, which melts nea ...
.
 The HDDA +2cycloaddition can occur via either a
The HDDA +2cycloaddition can occur via either a concerted
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rate
The ...
pathway or a stepwise reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
, diradical pathway. These two pathways can differ in activation energy depending on substrate and reaction system. Computational studies have suggested that while both pathways are comparable in activation energy for unactivated (unsubstituted) diynophiles, the stepwise pathway has a lower activation energy barrier, and so is the dominant pathway, for activated diynophiles.Liang, Y.; Hong, X.; Yu, P.; Houk, K. N. ''Org. Lett.'', 2014, ''16'', 570/ref>
Regiochemistry
The regiochemistry of non-symmetrical HDDA-derived benzyne trapping can be explained by a combination of electronic and ring distortion effects. Computationally, the more obtuse angle (a) corresponds to the more electron deficient (δ+) benzyne carbon, leading to attack of the nucleophilic component at this site. Consequently, the electrophilic component adds at the more electron rich (δ-) site (b).
Terminology
The HDDA reaction is a derivative of, and mechanistically related to, the classical Diels–Alder reaction. As described by Hoye and coworkers, the HDDA reaction can be viewed conceptually as a member of a series ofpericyclic reactions
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted reaction, concerted fashion, and the Localized molecular orbita ...
with increasing unsaturation (by incremental removal of hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
pairs). The “hexadehydro” descriptor is derived from this interpretation, as the simplest HDDA reaction product (o-benzyne, 4 hydrogens) has 6 fewer hydrogen atoms than the simplest Diels–Alder reaction product (cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon.
Prod ...
, 10 hydrogens).
 Formally, the hexadehydro Diels–Alder reaction describes only the formation of the benzyne, but this species is an unstable intermediate that reacts readily with a variety of trapping partners, including reaction
Formally, the hexadehydro Diels–Alder reaction describes only the formation of the benzyne, but this species is an unstable intermediate that reacts readily with a variety of trapping partners, including reaction solvents
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
. Thus, in practice the HDDA reaction describes a two-step cascade reaction of benzyne formation and trapping to yield the final product.
Historical development
The first examples of the HDDA reaction were reported independently in 1997 by the groups of Ueda and Johnson.Bradley, A. Z.; Johnson, R. P. ''J. Am. Chem. Soc.'', 1997, ''119'', 991/ref>Miyawaki, K.; Suzuki, R.; Kawano, T.; Ueda, I. ''Tetrahedron Lett.'', 1997, ''38'', 394
/ref> Johnson and co-workers observed the cyclization of 1,3,8-nonatriyne under pyrolysis, flash vacuum thermolysis (600 °C, 10−2 torr) to form two products,
indane
Indane or indan is an organic compound with the formula C9H10. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of inde ...
and the dehydrogenation product indene
Indene is an aromatic, polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of i ...
, in 95% combined yield. Deuterium labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' b ...
studies suggested that the product was formed by a +2cycloaddition to a benzyne intermediate, followed by in-situ reduction to form the observed products. Ueda and co-workers observed that acyclic tetraynes cyclized at room temperature to form 5H-fluorenol derivatives. The formation of a benzyne intermediate was determined by trapping studies, using benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
or anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintil ...
to trap the benzyne as a Diels–Alder adduct. Ueda and co-workers further elaborated on this method in subsequent reports, trapping the benzyne using a variety of nucleophiles (oxygen, nitrogen, and sulfur-based), as well as synthesizing larger, fused-ring aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
systems.
While known for over a decade, the HDDA reaction did not come into wider synthetic use until 2012, when Hoye and co-workers conducted a thorough investigation into the scope and utility of this cycloaddition. That paper referred to this diyne–diynophile reaction as the''“hexadehydro Diels–Alder (HDDA)'' reaction, and this terminology has since come into more widespread use. Since 2012, the HDDA reaction has been an area of renewed interest and has attracted further study by a number of research groups.Karmakar, R.; Mamidipalli, P.; Yun, S. Y.; Lee, D. ''Org. Lett.'', 2013, ''15'', 193/ref>
Reaction scope
One of the main advantages of the HDDA reaction over other methods of accessing benzynes is the simplicity of the reaction system. HDDA reaction of triynes or tetraynes forms benzynes without the direct formation of by-products. In comparison, the formation of benzyne through removal of ortho-substituents on arenes results in stoichiometric amounts of byproducts from those substituents. For example, formation of benzyne from 1 mole of 2-trimethylsilylphenyltrifluoromethanesulfonate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ...
(triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
) produces 1 mole of trimethylsilyl fluoride and 1 mole of triflate ion. Byproducts can compete with other reagents for benzyne trapping, cause side-reactions, and may require additional purification.
Additionally, the HDDA reaction can be useful for substrates with sensitive functionality that might not be tolerated by other benzyne formation conditions (e.g. strong base). The thermally-initiated HDDA reaction has been shown to tolerate esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
, ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
, protected amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a p ...
, ethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ r ...
, protected amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
, aryl halide
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, br ...
s, alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
, alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, and cyclopropanes Cyclopropanes are a family of organic compounds containing the cyclopropyl group. The parent is cyclopropane ().
Synthesis and reactions
Most cyclopropanes are not prepared from the parent cyclopropane, which is somewhat inert. Instead, yclopropy ...
.
Green chemistry
The HDDA reaction can fulfill several principles ofgreen chemistry
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. Wh ...
.
* Atom economy – All of the atoms in the HDDA substrate remain in the product after the reaction and atoms of the trapping reagent are incorporated into the product.
* Reduced waste – Formation of the benzyne species produces no stoichiometric byproducts. Products are often formed in high yield with few side-products.
* Catalysis – HDDA reaction occurs thermally or with a sub-stoichiometric amount of catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
.
Synthetic applications
Intramolecular trapping
The HDDA reaction can be used to synthesize multi-cyclic ring systems from linear precursors containing the diyne, diynophile, and the trapping group. For example, Hoye and co-workers were able to synthesize fused, tricyclic ring systems from linear triyne precursors in one step and high yields via a thermally-initiated, intramolecular HDDA reaction. Furthermore, both nitrogen- and oxygen-containing heterocycles could be incorporated by use of an appropriate precursor. In this case, the pendant silyl ether provided the trapping group, through a retro-Brook rearrangement
In organic chemistry the Brook rearrangement refers to any ,''n''carbon to oxygen silyl migration. The Rearrangement reaction, rearrangement was first observed in the late 1950s by Canadian chemist Adrian Gibbs Brook (1924–2013), after which ...
.

Intermolecular trapping
HDDA-generated benzynes can also be trapped intermolecularly by a variety of trapping reagents. Careful choice of trapping reagent can add further functionality, including aryl halides, aryl heteroatoms (phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
and aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
derivatives), and multiple ring systems.Niu, D.; Wang, T.; Woods, B. P.; Hoye, T. R. ''Org. Lett.'', 2014, ''16'', 25/ref>

Ene reactions
The HDDA reaction can be used in a cascade reaction sequence withene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile) ...
s, such as the Alder ene reaction and the aromatic ene reaction.Niu, D.; Hoye, T. R. ''Nat. Chem.'', 2014, ''6'', 34/ref> The HDDA-generated benzyne can be trapped with a suitable ene donor that is covalently tethered to the benzyne. The benzyne serves as the enophile, while the ene can be an alkene (Alder ene) or an aromatic ring (aromatic ene). Lee and co-workers have shown an HDDA-Alder ene cascade reaction that can produce a variety of products, including medium-sized fused rings, spirocycles, and
allenes
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is H or some organyl group). Allenes are classified as cumulated dienes. The parent compound o ...
.
 Hoye and co-workers demonstrated a thermally-initiated triple HDDA-aromatic ene-Alder ene cascade that leads to heavily functionalized products in one-step with no additional reagents or by-products.
Hoye and co-workers demonstrated a thermally-initiated triple HDDA-aromatic ene-Alder ene cascade that leads to heavily functionalized products in one-step with no additional reagents or by-products.

Dehydrogenation
HDDA-derived benzynes have also been shown to dehydrogenate saturatedalkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
to form alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
.Niu, D.; Willoughby, P. H.; Woods, B. P.; Baire, B.; Hoye, T. R. ''Nature'', 2013, 501, 53/ref> In the absence of external trapping reagents, the benzyne intermediate can abstract
vicinal (chemistry)
In chemistry the descriptor vicinal (from Latin ''vicinus'' = neighbor), abbreviated ''vic'', is a descriptor that identifies two functional groups as bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). It may arise from vicinal ...
hydrogen atoms from a suitable donor, often the reaction solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
(such as tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
or cyclooctane
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.
Cyclooctane has a camphoraceous odor.
Conformatio ...
). This desaturates the donor alkane, forming an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, and traps the benzyne to a dihydrobenzenoid product. Isotopic labelling and computational studies suggest that the double hydrogen transfer mechanism occurs by a concerted pathway and that the rate of reaction is highly dependent on the conformation of the alkane donor. This reaction can be used to access 1,2,3,4-tetrasubstituted aromatic rings, a substitution pattern that can be difficult to access through other synthetic methodology.

C-H activation
The HDDA reaction can also be used as a method of C-H activation, where a pendantalkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
C-H bond traps a metal-complexed aryne
In organic chemistry, arynes and benzynes are a class of highly Reactivity (chemistry), reactive chemical Chemical species, species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydro ...
intermediate. Lee and co-workers observed that transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
catalysts induced an HDDA reaction of tetraynes that was intramolecularly trapped by a pendant, sp3 C-H bond. Primary, secondary, and tertiary C-H bonds were all reactive trapping partners, with silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
salts being the most effective catalysts. Deuterium labelling experiments suggest that the (sp3) C-H bond breaking and (sp2) C-H bond forming reactions occur in a concerted fashion.

Fluorination
The silver-catalyzed HDDA reaction has also been used to synthesizeorganofluorine
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repell ...
compounds by use of a fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
-containing counterion
160px, cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.
In chemistry, a counterion (sometimes written as "counter ...
.Wang, K.-P.; Yun, S. Y.; Mamidipalli, P.; Lee, D. ''Chem. Sci.'', 2013, ''4'', 320/ref> The metal-complexed aryne intermediate can be trapped by the counterion to produce aryl rings with fluoro,
trifluoromethyl
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane ...
, or trifluoromethylthiol substituents. Unstable counterions, such as CF3−, can be produced in-situ.

The domino HDDA reaction
Properly designed polyyne substrate has been shown to undergo efficient cascade net +2cycloadditions merely upon being heated. This domino hexadehydro Diels–Alder reaction is initiated by a rate-limiting benzyne formation. Proceeding through naphthyne, anthracyne, and/or tetracyne intermediates, rapid bottom-up synthesis of highly fused, polycyclic aromatic compounds results.
The aza HDDA reaction
Nitriles can also participate in the HDDA reactions to generate pyridyne intermediates. ''In situ'' capturing of pyridynes gives rise to highly substituted and functionalized pyridine derivatives, which is complementary to other classical approaches for construction of this important class of heterocycles.
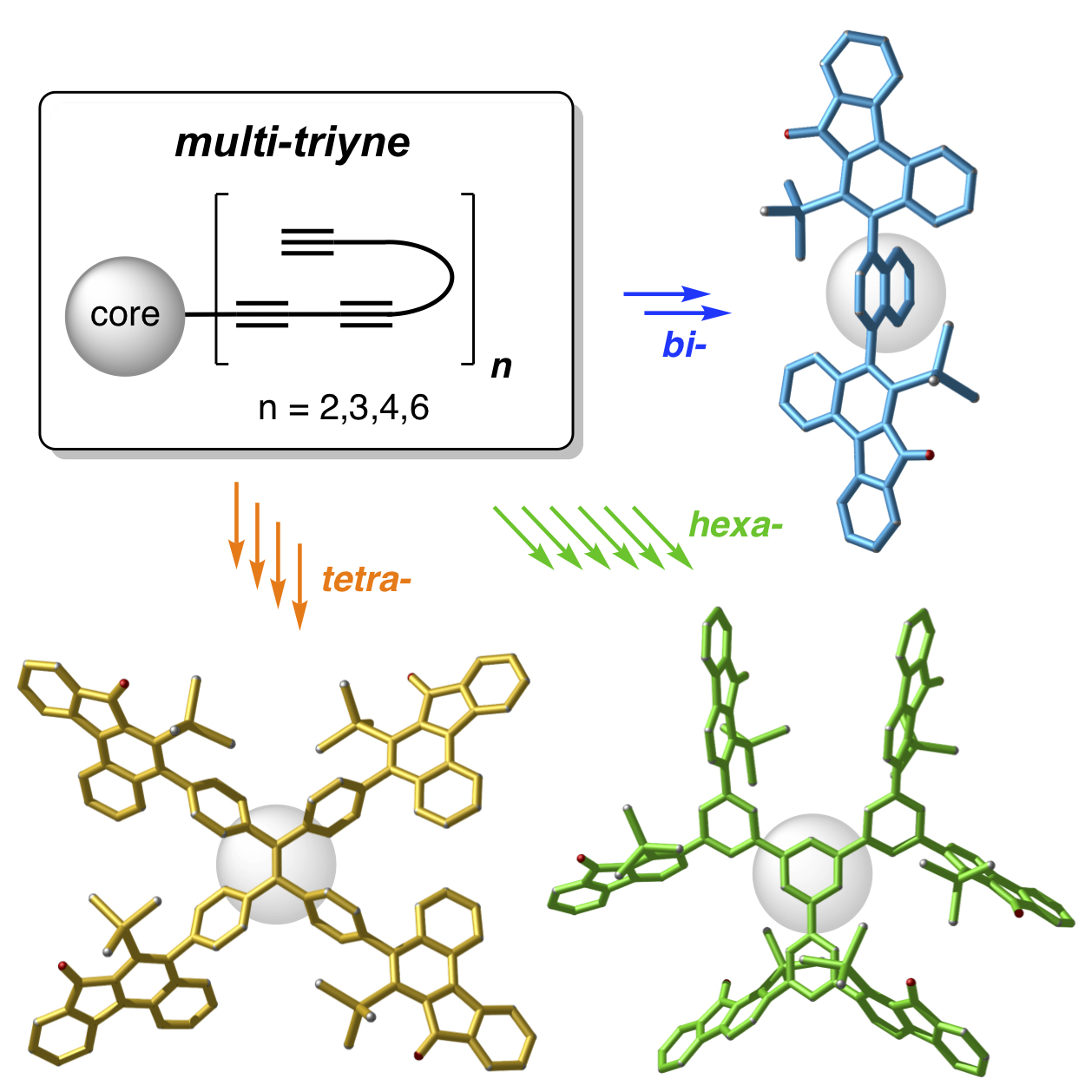
Radial HDDA reactions
Designer multi-ynes arrayed upon a common, central template undergo sequential, multiple cycloisomerization reactions to produce architecturally novel polycyclic compounds in a single operation. Diverse product topologies are accessible, ranging from highly fused, polycyclic aromatic compounds (PACs) to architectures having structurally complex arms adorning central phenylene or expanded phenylene cores.
References
{{DEFAULTSORT:Hexadehydro Diels-Alder reaction Cycloadditions Name reactions