Glutamic Protease on:
[Wikipedia]
[Google]
[Amazon]
Glutamic proteases are a group of
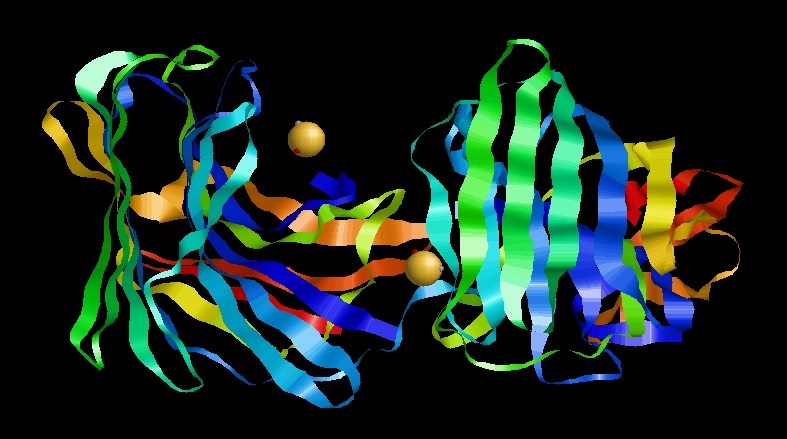 There are two independent
There are two independent
proteolytic
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s containing a glutamic acid residue within the active site. This type of protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
was first described in 2004 and became the sixth catalytic type of protease. Members of this group of protease had been previously assumed to be an aspartate protease
Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active ...
, but structural determination showed it to belong to a novel protease family. The first structure of this group of protease was scytalidoglutamic peptidase, the active site of which contains a catalytic dyad, glutamic acid (E) and glutamine (Q), which give rise to the name eqolisin. This group of proteases are found primarily in pathogenic fungi affecting plant and human.
Distribution and types
 There are two independent
There are two independent families
Family (from la, familia) is a group of people related either by consanguinity (by recognized birth) or affinity (by marriage or other relationship). The purpose of the family is to maintain the well-being of its members and of society. Ideal ...
of glutamic proteases (G1 and G2), and have a limited distribution. They were originally thought to be limited to filamentous fungi mainly in the Ascomycota phylum. Subsequently, however, glutamic proteases have been identified in bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
and archaea. A glutamic protease from a plant virus strawberry mottle virus has also been identified.
The first superfamily of glutamic proteases was identified in the fungi '' Scytalidium lignicola'' and ''Aspergillus niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on de ...
var. macrosporus'', from which scytalidoglutamic peptidase (eqolisin) and aspergilloglutamic peptidase are derived respectively. These two proteases contain active site Glu and Gln residues and are grouped under MEROPS family
Family (from la, familia) is a group of people related either by consanguinity (by recognized birth) or affinity (by marriage or other relationship). The purpose of the family is to maintain the well-being of its members and of society. Idea ...
G1.
A convergently evolved glutamic peptidase, the pre-neck appendage protein (bacteriophage phi-29), uses a Glu and Asp dyad at the active site, and is classified as MEROPS family G2.
Properties
These enzymes are acid proteases; eqolisin for example is most active at pH 2.0 when casein is used as substrate. Eqolosins prefer bulky amino acid residues at the P1 site and small amino acid residues at the P1′ site. A characteristic of the protease is its insensitivity topepstatin
Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide containing the unusual amino acid statine (Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta- ...
and S-PI (acetyl pepstatin) and it was previously classed as "pepstatin-insensitive carboxyl proteinases". The other "pepstatin-insensitive carboxyl proteinases" belongs to subfamily of serine protease
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site.
They are found ubiquitously in both eukaryotes and prokaryotes. Seri ...
, serine-carboxyl protease (sedolisin) which was discovered in 2001. These proteases are also not inhibited by DAN (diazoacetyl-DL-norleucine methylester) (7) but may be inhibited by EPNP (1,2-epoxy-3-(''p''-nitrophenoxy) propane).
Active site and mechanism of catalysis
The active site of eqolosin contains a distinctive glutamic acid andglutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
catalytic dyad which are involved in substrate binding and catalysis. These residues act as a nucleophile, with the glutamic acid serving as a general acid in the first phase of the reaction, donating a proton to the carbonyl oxygen in the peptide bond of the substrate. One or two water molecules may be involved in the reaction supplying a hydroxyl group, and the glutamic acid further donates a proton to the amide nitrogen, resulting in breakage of the peptide bond. The glutamine then returns the glutamic acid to its initial state.
See also
*Aspartic protease
Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the activ ...
References
{{Portal bar, Biology, border=no Proteases EC 3.4.23