Glucose And Fructose on:
[Wikipedia]
[Google]
[Amazon]
Glucose is a simple
 Glucose is usually present in solid form as a
Glucose is usually present in solid form as a
 The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of
The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of
 Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously
sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
with the molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. Glucose is overall the most abundant monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
, a subcategory of carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s. Glucose is mainly made by plants
Plants are predominantly Photosynthesis, photosynthetic eukaryotes of the Kingdom (biology), kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all curr ...
and most algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
during photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
in cell wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mech ...
s, the most abundant carbohydrate in the world.
In energy metabolism
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of ...
, glucose is the most important source of energy in all organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
s. Glucose for metabolism is stored as a polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
, in plants mainly as starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
and amylopectin Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.
Plants store starch within specialized organelles called amylopl ...
, and in animals as glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
. Glucose circulates in the blood of animals as blood sugar
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blo ...
. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
Mechanism
The ...
.
Glucose, as intravenous sugar solution
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutri ...
, is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health ...
. It is also on the list in combination with sodium chloride.
The name glucose is derived from Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic peri ...
(, "wine, must"), from (, "sweet"). The suffix "-ose
The suffix -ose ( or ) is used in biochemistry to form the names of sugars. This Latin suffix means "full of", "abounding in", "given to", or "like". Numerous systems exist to name specific sugars more descriptively.
Monosaccharides, the simplest ...
" is a chemical classifier, denoting a sugar.
History
Glucose was first isolated fromraisin
A raisin is a dried grape. Raisins are produced in many regions of the world and may be eaten raw or used in cooking, baking, and brewing. In the United Kingdom, Ireland, New Zealand, and Australia, the word ''raisin'' is reserved for the d ...
s in 1747 by the German chemist Andreas Marggraf. Glucose was discovered in grapes by another German chemistJohann Tobias Lowitz
Johann Tobias Lowitz (russian: Товий Егорович Ловиц 25 April 1757 – 7 December 1804) was a German-Russian chemist and pharmacist. He was among the first to notice the clarification of liquids by the use of charcoal for adsorpti ...
in 1792, and distinguished as being different from cane sugar (sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
). Glucose is the term coined by Jean Baptiste Dumas
Jean Baptiste André Dumas (14 July 180010 April 1884) was a French chemist, best known for his works on organic analysis and synthesis, as well as the determination of atomic weights (relative atomic masses) and molecular weights by measuring v ...
in 1838, which has prevailed in the chemical literature. Friedrich August Kekulé Friedrich may refer to:
Names
* Friedrich (surname), people with the surname ''Friedrich''
* Friedrich (given name), people with the given name ''Friedrich''
Other
* Friedrich (board game), a board game about Frederick the Great and the Seven Year ...
proposed the term dextrose (from the Latin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the ...
, meaning "right"), because in aqueous solution of glucose, the plane of linearly polarized light is turned to the right. In contrast, -fructose (a ketohexose) and -glucose turn linearly polarized light to the left. The earlier notation according to the rotation of the plane of linearly polarized light (''d'' and ''l''-nomenclature) was later abandoned in favor of the - and -notation, which refers to the absolute configuration of the asymmetric center farthest from the carbonyl group, and in concordance with the configuration of - or -glyceraldehyde.John F. Robyt: ''Essentials of Carbohydrate Chemistry.'' Springer Science & Business Media, 2012, . p. 7.
Since glucose is a basic necessity of many organisms, a correct understanding of its chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
makeup and structure contributed greatly to a general advancement in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
. This understanding occurred largely as a result of the investigations of Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of dra ...
, a German chemist who received the 1902 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
for his findings. The synthesis of glucose established the structure of organic material and consequently formed the first definitive validation of Jacobus Henricus van 't Hoff's theories of chemical kinetics and the arrangements of chemical bonds in carbon-bearing molecules. Between 1891 and 1894, Fischer established the stereochemical configuration of all the known sugars and correctly predicted the possible isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s, applying Van 't Hoff's theory of asymmetrical carbon atoms. The names initially referred to the natural substances. Their enantiomers were given the same name with the introduction of systematic nomenclatures, taking into account absolute stereochemistry (e.g. Fischer nomenclature, / nomenclature).
For the discovery of the metabolism of glucose Otto Meyerhof
Otto Fritz Meyerhof (; April 12, 1884 – October 6, 1951) was a German physician and biochemist who won the 1922 Nobel Prize in Physiology and Medicine.
Biography
Otto Fritz Meyerhof was born in Hannover, at Theaterplatz 16A (now:Rathenaustrasse ...
received the Nobel Prize in Physiology or Medicine
The Nobel Prize in Physiology or Medicine is awarded yearly by the Nobel Assembly at the Karolinska Institute for outstanding discoveries in physiology or medicine. The Nobel Prize is not a single prize, but five separate prizes that, accord ...
in 1922. Hans von Euler-Chelpin
Hans Karl August Simon von Euler-Chelpin (15 February 1873 – 6 November 1964) was a German-born Swedish biochemist. He won the Nobel Prize in Chemistry in 1929 with Arthur Harden for their investigations on the fermentation of sugar and enz ...
was awarded the Nobel Prize in Chemistry along with Arthur Harden
Sir Arthur Harden, FRS (12 October 1865 – 17 June 1940) was a British biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and ferment ...
in 1929 for their "research on the fermentation of sugar and their share of enzymes in this process". In 1947, Bernardo Houssay
Bernardo Alberto Houssay (April 10, 1887 – September 21, 1971) was an Argentine physiologist. Houssay was a co-recipient of the 1947 Nobel Prize for Physiology or Medicine for discovering the role played by pituitary hormones in regulating th ...
(for his discovery of the role of the pituitary gland in the metabolism of glucose and the derived carbohydrates) as well as Carl Carl may refer to:
*Carl, Georgia, city in USA
*Carl, West Virginia, an unincorporated community
* Carl (name), includes info about the name, variations of the name, and a list of people with the name
*Carl², a TV series
* "Carl", an episode of te ...
and Gerty Cori
Gerty Theresa Cori (; August 15, 1896 – October 26, 1957) was an Austro-Hungarian and American biochemist who in 1947 was the third woman to win a Nobel Prize in science, and the first woman to be awarded the Nobel Prize in Physiology or Me ...
(for their discovery of the conversion of glycogen from glucose) received the Nobel Prize in Physiology or Medicine. In 1970, Luis Leloir
Luis Federico Leloir (September 6, 1906 – December 2, 1987) was an Argentine physician and biochemist who received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways in lactose. Although born in France, Leloir r ...
was awarded the Nobel Prize in Chemistry for the discovery of glucose-derived sugar nucleotides in the biosynthesis of carbohydrates.
Chemical and physical properties
Glucose forms white or colorless solids that are highlysoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
in water and acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
but poorly soluble in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
. They melt at (''α'') and (''β''), and decompose
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ...
starting at with release of various volatile products, ultimately leaving a residue of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
.Wenyue Kang and Zhijun Zhang (2020): "Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts", ''Catalysts'', volume 10, pages 502-515. Glucose has a pK value of 12.16 at in water.
With six carbon atoms, it is classed as a hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
, a subcategory of the monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
s. -Glucose is one of the sixteen aldohexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s. The -isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
, -glucose, also known as ''dextrose'', occurs widely in nature, but the -isomer, -glucose, does not. Glucose can be obtained by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of carbohydrates such as milk sugar (lactose
Lactose is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from ' (gen. '), the Latin word for milk, plus the suffix '' - ...
), cane sugar (sucrose), maltose
}
Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two- ...
, cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
, glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
, etc. Dextrose is commonly commercially manufactured from cornstarch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch
Tapioca (; ) is a starch extracted from the storage roots of the cassava plant (''Manihot esculenta,'' also known as manioc), a species native to the North and Northeast regions of Brazil, but whose use is now spread throughout South America. ...
in tropical areas. The manufacturing process uses hydrolysis via pressurized steaming at controlled pH in a jet followed by further enzymatic depolymerization. Unbonded glucose is one of the main ingredients of honey
Honey is a sweet and viscous substance made by several bees, the best-known of which are honey bees. Honey is made and stored to nourish bee colonies. Bees produce honey by gathering and then refining the sugary secretions of plants (primar ...
.
Structure and nomenclature
 Glucose is usually present in solid form as a
Glucose is usually present in solid form as a monohydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was underst ...
with a closed pyran
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the ...
ring (dextrose hydrate). In aqueous solution, on the other hand, it is an open-chain to a small extent and is present predominantly as α- or β-pyranose Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity ...
, which interconvert. From aqueous solutions, the three known forms can be crystallized: α-glucopyranose, β-glucopyranose and β-glucopyranose hydrate. Glucose is a building block of the disaccharides lactose and sucrose (cane or beet sugar), of oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sugar ...
s such as raffinose
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by ...
and of polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
s such as starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
, amylopectin Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.
Plants store starch within specialized organelles called amylopl ...
, glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
, and cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
. The glass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubb ...
of glucose is and the Gordon–Taylor constant (an experimentally determined constant for the prediction of the glass transition temperature for different mass fractions of a mixture of two substances) is 4.5.Benjamin Caballero, Paul Finglas, Fidel Toldrá: ''Encyclopedia of Food and Health''. Academic Press (2016). , Volume 1, p. 76.
Open-chain form
The open-chain form of glucose makes up less than 0.02% of the glucose molecules in an aqueous solution. The rest is one of two cyclic hemiacetal forms. In itsopen-chain
In chemistry, an open-chain compound (also spelled as open chain compound) or acyclic compound (Greek prefix "α", ''without'' and "κύκλος", ''cycle'') is a compound with a linear structure, rather than a cyclic one.
An open-chain compound h ...
form, the glucose molecule has an open (as opposed to cyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in soc ...
) unbranched backbone of six carbon atoms, where C-1 is part of an aldehyde group
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
. Therefore, glucose is also classified as an aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from keto ...
, or an aldohexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
. The aldehyde group makes glucose a reducing sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a react ...
giving a positive reaction with the Fehling test
In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone () functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' r ...
.
Cyclic forms
In solutions, the open-chain form of glucose (either "-" or "-") exists in equilibrium with several cyclic isomers, each containing a ring of carbons closed by one oxygen atom. In aqueous solution, however, more than 99% of glucose molecules exist aspyranose Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity ...
forms. The open-chain form is limited to about 0.25%, and furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, bu ...
forms exist in negligible amounts. The terms "glucose" and "-glucose" are generally used for these cyclic forms as well. The ring arises from the open-chain form by an intramolecular nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
reaction between the aldehyde group (at C-1) and either the C-4 or C-5 hydroxyl group, forming a hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemike ...
linkage, .
The reaction between C-1 and C-5 yields a six-membered heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
system called a pyranose, which is a monosaccharide sugar (hence "-ose") containing a derivatised pyran
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the ...
skeleton. The (much rarer) reaction between C-1 and C-4 yields a five-membered furanose ring, named after the cyclic ether furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
. In either case, each carbon in the ring has one hydrogen and one hydroxyl attached, except for the last carbon (C-4 or C-5) where the hydroxyl is replaced by the remainder of the open molecule (which is or respectively).
The ring-closing reaction can give two products, denoted "α-" and "β-". When a glucopyranose molecule is drawn in the Haworth projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. Haworth projection approximate the shapes of the actual mole ...
, the designation "α-" means that the hydroxyl group attached to C-1 and the group at C-5 lies on opposite sides of the ring's plane (a '' trans'' arrangement), while "β-" means that they are on the same side of the plane (a '' cis'' arrangement). Therefore, the open-chain isomer -glucose gives rise to four distinct cyclic isomers: α--glucopyranose, β--glucopyranose, α--glucofuranose, and β--glucofuranose. These five structures exist in equilibrium and interconvert, and the interconversion is much more rapid with acid catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
.
 The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of
The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occur ...
.
In the solid state, only the glucopyranose forms are observed.
Some derivatives of glucofuranose, such as 1,2-''O''-isopropylidene--glucofuranose are stable and can be obtained pure as crystalline solids. For example, reaction of α-D-glucose with ''para''-tolylboronic acid reforms the normal pyranose ring to yield the 4-fold ester α-D-glucofuranose-1,2:3,5-bis(''p''-tolylboronate).
Mutarotation
 Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
izes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation Mutarotation is the change in the ''optical rotation'' because of the change in the equilibrium between two anomers, when the corresponding stereocenters interconvert. Cyclic sugars show mutarotation as α and β anomeric forms interconvert.
The op ...
. Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the ''axial'' orientation instead ...
. Mutarotation is considerably slower at temperatures close to .
Optical activity
Whether in water or the solid form, -(+)-glucose isdextrorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
, meaning it will rotate the direction of polarized light
Polarization (also polarisation) is a property applying to transverse waves that specifies the geometrical orientation of the oscillations. In a transverse wave, the direction of the oscillation is perpendicular to the direction of motion of the ...
clockwise as seen looking toward the light source. The effect is due to the chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
of the molecules, and indeed the mirror-image isomer, -(−)-glucose, is levorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
(rotates polarized light counterclockwise) by the same amount. The strength of the effect is different for each of the five tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
s.
Note that the - prefix does not refer directly to the optical properties of the compound. It indicates that the C-5 chiral centre has the same handedness as that of -glyceraldehyde (which was so labelled because it is dextrorotatory). The fact that -glucose is dextrorotatory is a combined effect of its four chiral centres, not just of C-5; and indeed some of the other -aldohexoses are levorotatory.
The conversion between the two anomers can be observed in a polarimeter
A polarimeter is a scientific instrument used to measure the angle of rotation caused by passing polarized light through an optically active substance.Manfred Hesse, Herbert Meier, Bernd Zeeh, Stefan Bienz, Laurent Bigler, Thomas Fox: ''Spektroskopische Methoden in der organischen Chemie''. 8th revised Edition. Georg Thieme, 2011, , p. 34 (in German). When equilibrium has been reached after a certain time due to mutarotation, the angle of rotation is +52.7° mL/(dm·g). By adding acid or base, this transformation is much accelerated. The equilibration takes place via the open-chain aldehyde form.
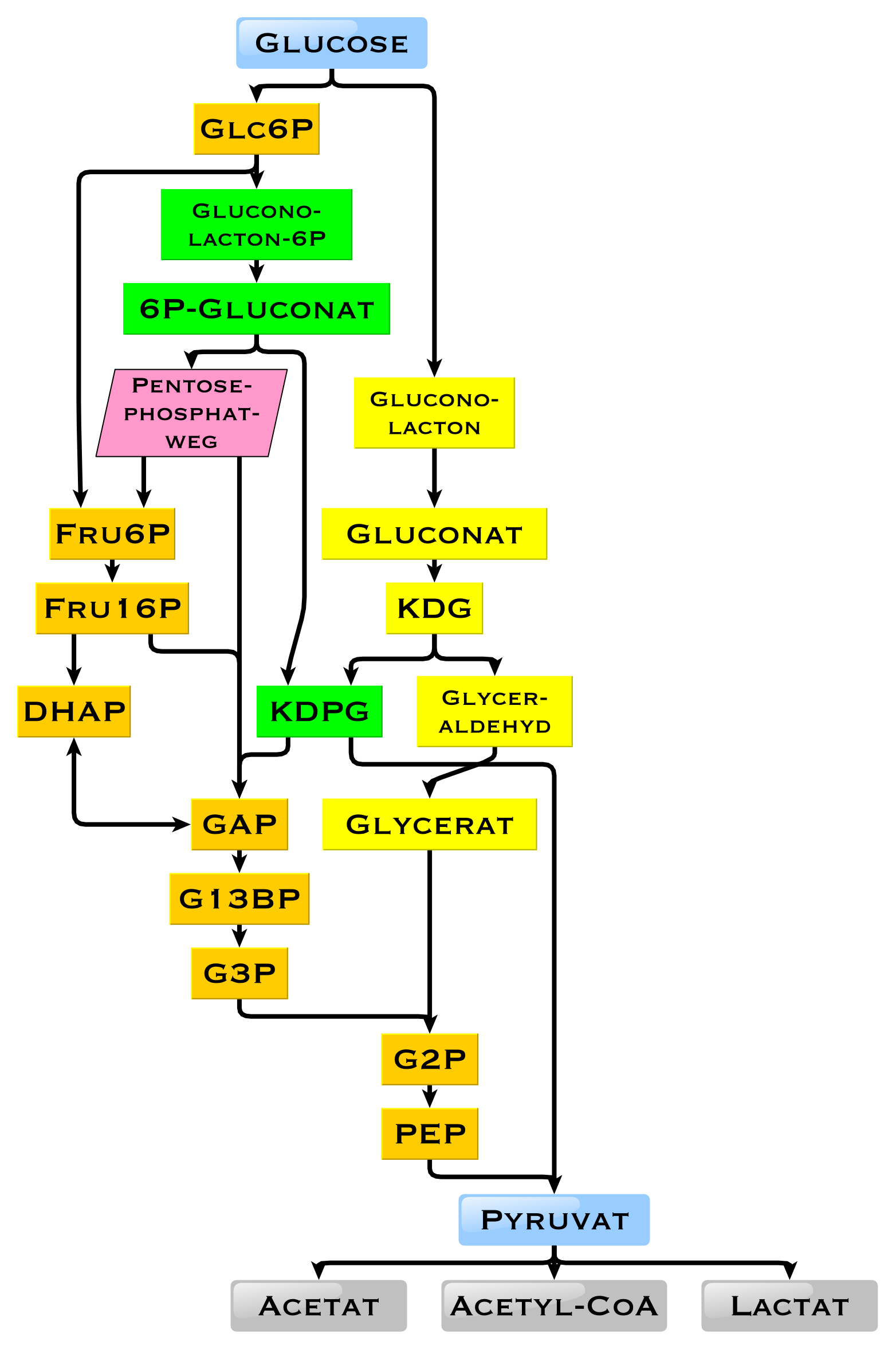
 In humans, glucose is metabolized by glycolysis and the pentose phosphate pathway.H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn: ''Biochemie''. Pearson Studium; 4. aktualisierte Auflage 2008; ; p. 490–496. (german) Glycolysis is used by all living organisms,Brian K. Hall: ''Strickberger's Evolution.'' Jones & Bartlett Publishers, 2013, , p. 164. with small variations, and all organisms generate energy from the breakdown of monosaccharides. In the further course of the metabolism, it can be completely degraded via oxidative decarboxylation, the citric acid cycle (synonym ''Krebs cycle'') and the respiratory chain to water and carbon dioxide. If there is not enough oxygen available for this, the glucose degradation in animals occurs anaerobic to lactate via lactic acid fermentation and releases much less energy. Muscular lactate enters the liver through the bloodstream in mammals, where gluconeogenesis occurs (Cori cycle). With a high supply of glucose, the metabolite acetyl-CoA from the Krebs cycle can also be used for fatty acid synthesis. Glucose is also used to replenish the body's glycogen stores, which are mainly found in liver and skeletal muscle. These processes are Hormone, hormonally regulated.
In other living organisms, other forms of fermentation can occur. The bacterium ''Escherichia coli'' can grow on nutrient media containing glucose as the sole carbon source. In some bacteria and, in modified form, also in archaea, glucose is degraded via the Entner-Doudoroff pathway.
Use of glucose as an energy source in cells is by either aerobic respiration, anaerobic respiration, or fermentation. The first step of glycolysis is the phosphorylation of glucose by a hexokinase to form glucose 6-phosphate. The main reason for the immediate phosphorylation of glucose is to prevent its diffusion out of the cell as the charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane. Furthermore, addition of the high-energy phosphate group Activation#Biochemistry, activates glucose for subsequent breakdown in later steps of glycolysis. At physiological conditions, this initial reaction is irreversible.
In anaerobic respiration, one glucose molecule produces a net gain of two ATP molecules (four ATP molecules are produced during glycolysis through substrate-level phosphorylation, but two are required by enzymes used during the process). In aerobic respiration, a molecule of glucose is much more profitable in that a maximum net production of 30 or 32 ATP molecules (depending on the organism) is generated,.
Tumor cells often grow comparatively quickly and consume an above-average amount of glucose by glycolysis, which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen. This is called the Warburg effect (oncology), Warburg effect. For the increased uptake of glucose in tumors various SGLT and GLUT are overly produced.
In yeast, ethanol is fermented at high glucose concentrations, even in the presence of oxygen (which normally leads to respiration rather than fermentation). This is called the Crabtree effect.
Glucose can also degrade to form carbon dioxide through abiotic means. This has been demonstrated to occur experimentally via oxidation and hydrolysis at 22˚C and a pH of 2.5.
In humans, glucose is metabolized by glycolysis and the pentose phosphate pathway.H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn: ''Biochemie''. Pearson Studium; 4. aktualisierte Auflage 2008; ; p. 490–496. (german) Glycolysis is used by all living organisms,Brian K. Hall: ''Strickberger's Evolution.'' Jones & Bartlett Publishers, 2013, , p. 164. with small variations, and all organisms generate energy from the breakdown of monosaccharides. In the further course of the metabolism, it can be completely degraded via oxidative decarboxylation, the citric acid cycle (synonym ''Krebs cycle'') and the respiratory chain to water and carbon dioxide. If there is not enough oxygen available for this, the glucose degradation in animals occurs anaerobic to lactate via lactic acid fermentation and releases much less energy. Muscular lactate enters the liver through the bloodstream in mammals, where gluconeogenesis occurs (Cori cycle). With a high supply of glucose, the metabolite acetyl-CoA from the Krebs cycle can also be used for fatty acid synthesis. Glucose is also used to replenish the body's glycogen stores, which are mainly found in liver and skeletal muscle. These processes are Hormone, hormonally regulated.
In other living organisms, other forms of fermentation can occur. The bacterium ''Escherichia coli'' can grow on nutrient media containing glucose as the sole carbon source. In some bacteria and, in modified form, also in archaea, glucose is degraded via the Entner-Doudoroff pathway.
Use of glucose as an energy source in cells is by either aerobic respiration, anaerobic respiration, or fermentation. The first step of glycolysis is the phosphorylation of glucose by a hexokinase to form glucose 6-phosphate. The main reason for the immediate phosphorylation of glucose is to prevent its diffusion out of the cell as the charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane. Furthermore, addition of the high-energy phosphate group Activation#Biochemistry, activates glucose for subsequent breakdown in later steps of glycolysis. At physiological conditions, this initial reaction is irreversible.
In anaerobic respiration, one glucose molecule produces a net gain of two ATP molecules (four ATP molecules are produced during glycolysis through substrate-level phosphorylation, but two are required by enzymes used during the process). In aerobic respiration, a molecule of glucose is much more profitable in that a maximum net production of 30 or 32 ATP molecules (depending on the organism) is generated,.
Tumor cells often grow comparatively quickly and consume an above-average amount of glucose by glycolysis, which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen. This is called the Warburg effect (oncology), Warburg effect. For the increased uptake of glucose in tumors various SGLT and GLUT are overly produced.
In yeast, ethanol is fermented at high glucose concentrations, even in the presence of oxygen (which normally leads to respiration rather than fermentation). This is called the Crabtree effect.
Glucose can also degrade to form carbon dioxide through abiotic means. This has been demonstrated to occur experimentally via oxidation and hydrolysis at 22˚C and a pH of 2.5.
 Glucose is a ubiquitous fuel in biology. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration (in bacteria), or Fermentation (biochemistry), fermentation. Glucose is the human body's key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g., starch) yields monosaccharide, mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form carbon dioxide and water, yielding energy mostly in the form of adenosine triphosphate, ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is 16.2 kilojoules per gramGeorg Schwedt: ''Zuckersüße Chemie.'' John Wiley & Sons, 2012, , p. 100 . or 15.7 kJ/g (3.74 kcal/g). The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage. Differences exist in which end product can no longer be used for energy production. The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain, so its availability influences psychological processes. When Hypoglycaemia, glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired. In the brain, which is dependent on glucose and oxygen as the major source of energy, the glucose concentration is usually 4 to 6 mM (5 mM equals 90 mg/dL), but decreases to 2 to 3 mM when fasting. Confusion occurs below 1 mM and coma at lower levels.
The glucose in the blood is called
Glucose is a ubiquitous fuel in biology. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration (in bacteria), or Fermentation (biochemistry), fermentation. Glucose is the human body's key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g., starch) yields monosaccharide, mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form carbon dioxide and water, yielding energy mostly in the form of adenosine triphosphate, ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is 16.2 kilojoules per gramGeorg Schwedt: ''Zuckersüße Chemie.'' John Wiley & Sons, 2012, , p. 100 . or 15.7 kJ/g (3.74 kcal/g). The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage. Differences exist in which end product can no longer be used for energy production. The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain, so its availability influences psychological processes. When Hypoglycaemia, glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired. In the brain, which is dependent on glucose and oxygen as the major source of energy, the glucose concentration is usually 4 to 6 mM (5 mM equals 90 mg/dL), but decreases to 2 to 3 mM when fasting. Confusion occurs below 1 mM and coma at lower levels.
The glucose in the blood is called
 Individuals with diabetes or other conditions that result in hypoglycemia, low blood sugar often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder), hard candy, or sugar packet.
Individuals with diabetes or other conditions that result in hypoglycemia, low blood sugar often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder), hard candy, or sugar packet.
 Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
 Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candy, candies, toffee and fondant icing, fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are caramelization and, in presence of amino acids, the Maillard reaction.
In addition, various organic acids can be biotechnologically produced from glucose, for example by fermentation with ''Clostridium thermoaceticum'' to produce
Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candy, candies, toffee and fondant icing, fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are caramelization and, in presence of amino acids, the Maillard reaction.
In addition, various organic acids can be biotechnologically produced from glucose, for example by fermentation with ''Clostridium thermoaceticum'' to produce
wayback=20100331071121 ''Anlagen zum Positionspapier der Fachgruppe Nuklearchemie''
, February 2000. where it is by far the most commonly used diagnostic agent.
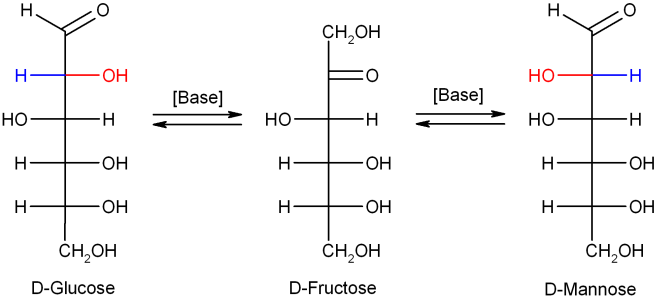
Isomerisation
In dilutesodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
or other dilute bases, the monosaccharides mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation ...
, glucose and fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
interconvert (via a Lobry de Bruyn–Alberda–Van Ekenstein transformation), so that a balance between these isomers is formed. This reaction proceeds via an enediol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The ter ...
:

Biochemical properties
Glucose is the most abundant monosaccharide. Glucose is also the most widely used aldohexose in most living organisms. One possible explanation for this is that glucose has a lower tendency than other aldohexoses to react nonspecifically with theamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
groups of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s. This reaction—glycation
Glycation (sometimes called non-enzymatic glycosylation) is the covalent attachment of a sugar to a protein or lipid. Typical sugars that participate in glycation are glucose, fructose, and their derivatives. Glycation is the non-enzymatic proces ...
—impairs or destroys the function of many proteins, e.g. in glycated hemoglobin
Glycated hemoglobin, also known as HbA1c, glycohemoglobin, hemoglobin A1c, A1C, is a form of hemoglobin (Hb) that is chemically linked to a sugar. Most monosaccharides, including glucose, galactose and fructose, spontaneously (i.e. non-enzymatic ...
. Glucose's low rate of glycation can be attributed to its having a more stable cyclic form
Cyclic form is a technique of musical construction, involving multiple sections or movements, in which a theme, melody, or thematic material occurs in more than one movement as a unifying device. Sometimes a theme may occur at the beginning and e ...
compared to other aldohexoses, which means it spends less time than they do in its reactive open-chain form. The reason for glucose having the most stable cyclic form of all the aldohexoses is that its hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
s (with the exception of the hydroxy group on the anomeric carbon of -glucose) are in the equatorial position. Presumably, glucose is the most abundant natural monosaccharide because it is less glycated with proteins than other monosaccharides.Jeremy M. Berg: ''Stryer Biochemie.'' Springer-Verlag, 2017, , p. 531. (german) Another hypothesis is that glucose, being the only -aldohexose that has all five hydroxy substituents in the equatorial Equatorial may refer to something related to:
*Earth's equator
**the tropics, the Earth's equatorial region
**tropical climate
*the Celestial equator
** equatorial orbit
**equatorial coordinate system
** equatorial mount, of telescopes
* equatorial ...
position in the form of β--glucose, is more readily accessible to chemical reactions, for example, for esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
or acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
formation. For this reason, -glucose is also a highly preferred building block in natural polysaccharides (glycans). Polysaccharides that are composed solely of glucose are termed glucan
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of ...
s.
Glucose is produced by plants through photosynthesis using sunlight, water and carbon dioxide and can be used by all living organisms as an energy and carbon source. However, most glucose does not occur in its free form, but in the form of its polymers, i.e. lactose, sucrose, starch and others which are energy reserve substances, and cellulose and chitin
Chitin ( C8 H13 O5 N)n ( ) is a long-chain polymer of ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chit ...
, which are components of the cell wall in plants or fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
and arthropods, respectively. These polymers, when consumed by animals, fungi and bacteria, are degraded to glucose using enzymes. All animals are also able to produce glucose themselves from certain precursors as the need arises. Neurons, cells of the renal medulla and erythrocytes depend on glucose for their energy production.Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 195. (german) In adult humans, there is about of glucose,U. Satyanarayana: ''Biochemistry.'' Elsevier Health Sciences, 2014, . p. 674. of which about is present in the blood. Approximately of glucose is produced in the liver of an adult in 24 hours.
Many of the long-term complications of diabetes (e.g., Visual impairment, blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. In contrast, enzyme-regulated addition of sugars to protein is called glycosylation and is essential for the function of many proteins.
Uptake
Ingested glucose initially binds to the receptor for sweet taste on the tongue in humans. This complex of the proteins T1R2 and T1R3 makes it possible to identify glucose-containing food sources. Glucose mainly comes from food—about per day is produced by conversion of food,Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 404. but it is also synthesized from other metabolites in the body's cells. In humans, the breakdown of glucose-containing polysaccharides happens in part already during chewing by means of amylase, which is contained in saliva, as well as by maltase, lactase, and sucrase on the brush border of the small intestine. Glucose is a building block of many carbohydrates and can be split off from them using certain enzymes. Glucosidases, a subgroup of the glycosidases, first catalyze the hydrolysis of long-chain glucose-containing polysaccharides, removing terminal glucose. In turn, disaccharides are mostly degraded by specific glycosidases to glucose. The names of the degrading enzymes are often derived from the particular poly- and disaccharide; inter alia, for the degradation of polysaccharide chains there are amylases (named after amylose, a component of starch), cellulases (named after cellulose), chitinases (named after chitin), and more. Furthermore, for the cleavage of disaccharides, there are maltase, lactase, sucrase, trehalase, and others. In humans, about 70 genes are known that code for glycosidases. They have functions in the digestion and degradation of glycogen, sphingolipids, mucopolysaccharides, and poly(Adenosine diphosphate ribose, ADP-ribose). Humans do not produce cellulases, chitinases, or trehalases, but the bacteria in the gut microbiota do. In order to get into or out of cell membranes of cells and membranes of cell compartments, glucose requires special transport proteins from the major facilitator superfamily. In the small intestine (more precisely, in the jejunum),Harold A. Harper: ''Medizinische Biochemie.'' Springer-Verlag, 2013, , p. 641. glucose is taken up into the intestinal epithelium with the help of glucose transporters via a secondary active transport mechanism called sodium ion-glucose symport via sodium/glucose cotransporter 1 (SGLT1). Further transfer occurs on the basolateral side of the intestinal epithelial cells via the glucose transporter GLUT2, as well uptake into hepatocyte, liver cells, kidney cells, cells of the Pancreatic islets, islets of Langerhans, neurons, astrocytes, and tanycytes. Glucose enters the liver via the portal vein and is stored there as a cellular glycogen. In the liver cell, it is Phosphorylation, phosphorylated by glucokinase at position 6 to form glucose 6-phosphate, which cannot leave the cell. Glucose 6-phosphatase can convert glucose 6-phosphate back into glucose exclusively in the liver, so the body can maintain a sufficient blood glucose concentration. In other cells, uptake happens by passive transport through one of the 14 GLUT proteins. In the other cell types, phosphorylation occurs through a hexokinase, whereupon glucose can no longer diffuse out of the cell. The glucose transporter GLUT1 is produced by most cell types and is of particular importance for nerve cells and pancreatic Beta cell, β-cells. GLUT3 is highly expressed in nerve cells. Glucose from the bloodstream is taken up by GLUT4 from muscle cells (of the skeletal muscle and heart muscle) and fat cells. GLUT14 is expressed exclusively in testicles. Excess glucose is broken down and converted into fatty acids, which are stored as triglycerides. In the kidneys, glucose in the urine is absorbed via SGLT1 and SGLT2 in the apical cell membranes and transmitted via GLUT2 in the basolateral cell membranes. About 90% of kidney glucose reabsorption is via SGLT2 and about 3% via SGLT1.Biosynthesis
In plants and some prokaryotes, glucose is a product ofphotosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
. Glucose is also formed by the breakdown of polymeric forms of glucose like glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
(in animals and mushrooms) or starch (in plants). The cleavage of glycogen is termed glycogenolysis, the cleavage of starch is called starch degradation.
The metabolic pathway that begins with molecules containing two to four carbon atoms (C) and ends in the glucose molecule containing six carbon atoms is called gluconeogenesis and occurs in all living organisms. The smaller starting materials are the result of other metabolic pathways. Ultimately almost all biomolecules come from the assimilation of carbon dioxide in plants and microbes during photosynthesis. The free energy of formation of α--glucose is 917.2 kilojoules per mole. In humans, gluconeogenesis occurs in the liver and kidney,Leszek Szablewski: ''Glucose Homeostasis and Insulin Resistance.'' Bentham Science Publishers, 2011, , p. 46. but also in other cell types. In the liver about of glycogen are stored, in skeletal muscle about .Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 389. However, the glucose released in muscle cells upon cleavage of the glycogen can not be delivered to the circulation because glucose is phosphorylated by the hexokinase, and a glucose-6-phosphatase is not expressed to remove the phosphate group. Unlike for glucose, there is no transport protein for Glucose-6-phosphate dehydrogenase (coenzyme-F420), glucose-6-phosphate. Gluconeogenesis allows the organism to build up glucose from other metabolites, including lactic acid, lactate or certain amino acids, while consuming energy. The renal tubular cells can also produce glucose.
Glucose also can be found outside of living organisms in the ambient environment. Glucose concentrations in the atmosphere are detected via collection of samples by aircraft and are known to vary from location to location. For example, glucose concentrations in atmospheric air from inland China range from 0.8-20.1 pg/L, whereas east coastal China glucose concentrations range from 10.3-142 pg/L.
Glucose degradation
Energy source
 Glucose is a ubiquitous fuel in biology. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration (in bacteria), or Fermentation (biochemistry), fermentation. Glucose is the human body's key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g., starch) yields monosaccharide, mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form carbon dioxide and water, yielding energy mostly in the form of adenosine triphosphate, ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is 16.2 kilojoules per gramGeorg Schwedt: ''Zuckersüße Chemie.'' John Wiley & Sons, 2012, , p. 100 . or 15.7 kJ/g (3.74 kcal/g). The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage. Differences exist in which end product can no longer be used for energy production. The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain, so its availability influences psychological processes. When Hypoglycaemia, glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired. In the brain, which is dependent on glucose and oxygen as the major source of energy, the glucose concentration is usually 4 to 6 mM (5 mM equals 90 mg/dL), but decreases to 2 to 3 mM when fasting. Confusion occurs below 1 mM and coma at lower levels.
The glucose in the blood is called
Glucose is a ubiquitous fuel in biology. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration (in bacteria), or Fermentation (biochemistry), fermentation. Glucose is the human body's key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g., starch) yields monosaccharide, mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form carbon dioxide and water, yielding energy mostly in the form of adenosine triphosphate, ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is 16.2 kilojoules per gramGeorg Schwedt: ''Zuckersüße Chemie.'' John Wiley & Sons, 2012, , p. 100 . or 15.7 kJ/g (3.74 kcal/g). The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage. Differences exist in which end product can no longer be used for energy production. The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain, so its availability influences psychological processes. When Hypoglycaemia, glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired. In the brain, which is dependent on glucose and oxygen as the major source of energy, the glucose concentration is usually 4 to 6 mM (5 mM equals 90 mg/dL), but decreases to 2 to 3 mM when fasting. Confusion occurs below 1 mM and coma at lower levels.
The glucose in the blood is called blood sugar
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blo ...
. Blood sugar levels are regulated by glucose-binding nerve cells in the hypothalamus. In addition, glucose in the brain binds to glucose receptors of the reward system in the nucleus accumbens. The binding of glucose to the sweet receptor on the tongue induces a release of various hormones of energy metabolism, either through glucose or through other sugars, leading to an increased cellular uptake and lower blood sugar levels. Artificial sweeteners do not lower blood sugar levels.
The blood sugar content of a healthy person in the short-time fasting state, e.g. after overnight fasting, is about 70 to 100 mg/dL of blood (4 to 5.5 mM). In blood plasma, the measured values are about 10–15% higher. In addition, the values in the artery, arterial blood are higher than the concentrations in the vein, venous blood since glucose is absorbed into the tissue during the passage of the capillary bed. Also in the capillary blood, which is often used for blood sugar determination, the values are sometimes higher than in the venous blood. The glucose content of the blood is regulated by the hormones insulin, incretin and glucagon. Insulin lowers the glucose level, glucagon increases it. Furthermore, the hormones adrenaline, thyroxine, glucocorticoids, somatotropin and adrenocorticotropin lead to an increase in the glucose level. There is also a hormone-independent regulation, which is referred to as glucose autoregulation. After food intake the blood sugar concentration increases. Values over 180 mg/dL in venous whole blood are pathological and are termed hyperglycemia, values below 40 mg/dL are termed hypoglycaemia. When needed, glucose is released into the bloodstream by glucose-6-phosphatase from glucose-6-phosphate originating from liver and kidney glycogen, thereby regulating the homeostasis of blood glucose concentration. In ruminants, the blood glucose concentration is lower (60 mg/dL in cattle and 40 mg/dL in sheep), because the carbohydrates are converted more by their gut microbiota into short-chain fatty acids.Harold A. Harper: ''Medizinische Biochemie''. Springer-Verlag, 2013, , p. 294.
Some glucose is converted to lactic acid by astrocytes, which is then utilized as an energy source by brain cells; some glucose is used by intestinal cells and red blood cells, while the rest reaches the liver, adipose tissue and muscle cells, where it is absorbed and stored as glycogen (under the influence of insulin). Liver cell glycogen can be converted to glucose and returned to the blood when insulin is low or absent; muscle cell glycogen is not returned to the blood because of a lack of enzymes. In Adipocyte, fat cells, glucose is used to power reactions that synthesize some fat types and have other purposes. Glycogen is the body's "glucose energy storage" mechanism, because it is much more "space efficient" and less reactive than glucose itself.
As a result of its importance in human health, glucose is an analyte in glucose tests that are common medical blood tests. Eating or fasting prior to taking a blood sample has an effect on analyses for glucose in the blood; a high fasting glucose blood sugar
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blo ...
level may be a sign of prediabetes or diabetes mellitus.
The glycemic index is an indicator of the speed of resorption and conversion to blood glucose levels from ingested carbohydrates, measured as the area under a curve, area under the curve of blood glucose levels after consumption in comparison to glucose (glucose is defined as 100).Richard A. Harvey, Denise R. Ferrier: ''Biochemistry''. 5th Edition, Lippincott Williams & Wilkins, 2011, , p. 366. The clinical importance of the glycemic index is controversial,U Satyanarayana: ''Biochemistry''. Elsevier Health Sciences, 2014, , p. 508. as foods with high fat contents slow the resorption of carbohydrates and lower the glycemic index, e.g. ice cream. An alternative indicator is the insulin index, measured as the impact of carbohydrate consumption on the blood insulin levels. The glycemic load is an indicator for the amount of glucose added to blood glucose levels after consumption, based on the glycemic index and the amount of consumed food.
Precursor
Organisms use glucose as a precursor for the synthesis of several important substances. Starch,cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
, and glycogen ("animal starch") are common glucose polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s (polysaccharides). Some of these polymers (starch or glycogen) serve as energy stores, while others (cellulose and chitin
Chitin ( C8 H13 O5 N)n ( ) is a long-chain polymer of ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chit ...
, which is made from a derivative of glucose) have structural roles. Oligosaccharides of glucose combined with other sugars serve as important energy stores. These include lactose, the predominant sugar in milk, which is a glucose-galactose disaccharide, and sucrose, another disaccharide which is composed of glucose and fructose. Glucose is also added onto certain proteins and lipids in a process called glycosylation. This is often critical for their functioning. The enzymes that join glucose to other molecules usually use phosphorylation, phosphorylated glucose to power the formation of the new bond by coupling it with the breaking of the glucose-phosphate bond.
Other than its direct use as a monomer, glucose can be broken down to synthesize a wide variety of other biomolecules. This is important, as glucose serves both as a primary store of energy and as a source of organic carbon. Glucose can be broken down and converted into lipids. It is also a precursor for the synthesis of other important molecules such as vitamin C (ascorbic acid). In living organisms, glucose is converted to several other chemical compounds that are the starting material for various metabolic pathways. Among them, all other monosaccharidesPeter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 27. such as fructose (via the polyol pathway),Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 199, 200. mannose (the epimer of glucose at position 2), galactose (the epimer at position 4), fucose, various uronic acids and the amino sugars are produced from glucose.Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 214. In addition to the phosphorylation to glucose-6-phosphate, which is part of the glycolysis, glucose can be oxidized during its degradation to glucono-1,5-lactone. Glucose is used in some bacteria as a building block in the trehalose or the dextran biosynthesis and in animals as a building block of glycogen. Glucose can also be converted from bacterial xylose isomerase to fructose. In addition, glucose metabolites produce all nonessential amino acids, sugar alcohols such as mannitol and sorbitol, fatty acids, cholesterol and nucleic acids. Finally, glucose is used as a building block in the glycosylation of proteins to glycoproteins, glycolipids, peptidoglycans, glycosides and other substances (catalyzed by glycosyltransferases) and can be cleaved from them by glycosidases.
Pathology
Diabetes
Diabetes is a metabolic disorder where the body is unable to regulate Blood sugar, levels of glucose in the blood either because of a lack of insulin in the body or the failure, by cells in the body, to respond properly to insulin. Each of these situations can be caused by persistently high elevations of blood glucose levels, through pancreatic burnout and insulin resistance. The pancreas is the organ responsible for the secretion of the hormones insulin and glucagon. Insulin is a hormone that regulates glucose levels, allowing the body's cells to absorb and use glucose. Without it, glucose cannot enter the cell and therefore cannot be used as fuel for the body's functions. If the pancreas is exposed to persistently high elevations of blood glucose levels, the beta cell, insulin-producing cells in the pancreas could be damaged, causing a lack of insulin in the body. Insulin resistance occurs when the pancreas tries to produce more and more insulin in response to persistently elevated blood glucose levels. Eventually, the rest of the body becomes resistant to the insulin that the pancreas is producing, thereby requiring more insulin to achieve the same blood glucose-lowering effect, and forcing the pancreas to produce even more insulin to compete with the resistance. This negative spiral contributes to pancreatic burnout, and the disease progression of diabetes. To monitor the body's response to blood glucose-lowering therapy, glucose levels can be measured. Blood glucose monitoring can be performed by multiple methods, such as the fasting glucose test which measures the level of glucose in the blood after 8 hours of fasting. Another test is the 2-hour glucose tolerance test (GTT)for this test, the person has a fasting glucose test done, then drinks a 75-gram glucose drink and is retested. This test measures the ability of the person's body to process glucose. Over time the blood glucose levels should decrease as insulin allows it to be taken up by cells and exit the blood stream.Hypoglycemia management
 Individuals with diabetes or other conditions that result in hypoglycemia, low blood sugar often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder), hard candy, or sugar packet.
Individuals with diabetes or other conditions that result in hypoglycemia, low blood sugar often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder), hard candy, or sugar packet.
Sources
 Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Commercial production
Glucose is produced industrially from starch by enzyme, enzymatichydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
using glucose amylase or by the use of acids. Enzymatic hydrolysis has largely displaced acid-catalyzed hydrolysis reactions.P. J. Fellows: ''Food Processing Technology. Woodhead Publishing'', 2016, , p. 197. The result is glucose syrup (enzymatically with more than 90% glucose in the dry matter) with an annual worldwide production volume of 20 million tonnes (as of 2011).Thomas Becker, Dietmar Breithaupt, Horst Werner Doelle, Armin Fiechter, Günther Schlegel, Sakayu Shimizu, Hideaki Yamada: ''Biotechnology'', in: ''Ullmann's Encyclopedia of Industrial Chemistry'', 7th Edition, Wiley-VCH, 2011. . Volume 6, p. 48. This is the reason for the former common name "starch sugar". The amylases most often come from ''Bacillus licheniformis''The Amylase Research Society of Japan: ''Handbook of Amylases and Related Enzymes.'' Elsevier, 2014, , p. 195. or ''Bacillus subtilis'' (strain MN-385), which are more thermostable than the originally used enzymes. Starting in 1982, pullulanases from ''Aspergillus niger'' were used in the production of glucose syrup to convert amylopectin to starch (amylose), thereby increasing the yield of glucose. The reaction is carried out at a pH = 4.6–5.2 and a temperature of 55–60 °C. Corn syrup has between 20% and 95% glucose in the dry matter. The Japanese form of the glucose syrup, Mizuame, is made from sweet potato or rice starch. Maltodextrin contains about 20% glucose.
Many crops can be used as the source of starch. Maize, rice, wheat, cassava, potato, barley, sweet potato,Alan Davidson: ''The Oxford Companion to Food''. OUP Oxford, 2014, , p. 527. corn husk and sago are all used in various parts of the world. In the United States, corn starch (from maize) is used almost exclusively. Some commercial glucose occurs as a component of invert sugar, a roughly 1:1 mixture of glucose and fructose that is produced from sucrose. In principle, cellulose could be hydrolyzed to glucose, but this process is not yet commercially practical.
Conversion to fructose
In the US, almost exclusively corn (more precisely, corn syrup) is used as glucose source for the production of isoglucose, which is a mixture of glucose and fructose, since fructose has a higher sweetening powerwith same physiological calorific value of 374 kilocalories per 100 g. The annual world production of isoglucose is 8 million tonnes (as of 2011). When made from corn syrup, the final product is high-fructose corn syrup (HFCS).Commercial usage
 Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candy, candies, toffee and fondant icing, fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are caramelization and, in presence of amino acids, the Maillard reaction.
In addition, various organic acids can be biotechnologically produced from glucose, for example by fermentation with ''Clostridium thermoaceticum'' to produce
Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candy, candies, toffee and fondant icing, fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are caramelization and, in presence of amino acids, the Maillard reaction.
In addition, various organic acids can be biotechnologically produced from glucose, for example by fermentation with ''Clostridium thermoaceticum'' to produce acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, with ''Penicillium notatum'' for the production of araboascorbic acid, with ''Rhizopus delemar'' for the production of fumaric acid, with ''Aspergillus niger'' for the production of gluconic acid, with ''Candida brumptii'' to produce isocitric acid, with ''Aspergillus terreus'' for the production of itaconic acid, with ''Pseudomonas fluorescens'' for the production of 2-ketogluconic acid, with ''Gluconobacter suboxydans'' for the production of 5-ketogluconic acid, with ''Aspergillus oryzae'' for the production of kojic acid, with ''Lactobacillus delbrueckii'' for the production of lactic acid, with ''Lactobacillus brevis'' for the production of malic acid, with ''Propionibacter shermanii'' for the production of propionic acid, with ''Pseudomonas aeruginosa'' for the production of pyruvic acid and with ''Gluconobacter suboxydans'' for the production of tartaric acid.James A. Kent: ''Riegel's Handbook of Industrial Chemistry''. Springer Science & Business Media, 2013, , p. 938. Potent, bioactive natural products like triptolide that inhibit mammalian transcription via inhibition of the XPB subunit of the general transcription factor TFIIH has been recently reported as a glucose conjugate for targeting hypoxic cancer cells with increased glucose transporter expression. Recently, glucose has been gaining commercial use as a key component of "kits" containing lactic acid and insulin intended to induce hypoglycemia and hyperlactatemia to combat different cancers and infections.
Analysis
When a glucose molecule is to be detected at a certain position in a larger molecule, nuclear magnetic resonance spectroscopy, X-ray crystallography analysis or lectin immunostaining is performed with concanavalin A reporter enzyme conjugate, which binds only glucose or mannose.Classical qualitative detection reactions
These reactions have only historical significance:Fehling test
TheFehling test
In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone () functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' r ...
is a classic method for the detection of aldoses.H. Fehling: ''Quantitative Bestimmung des Zuckers im Harn''. In: ''Archiv für physiologische Heilkunde'' (1848), volume 7, p. 64–73 (in German). Due to mutarotation, glucose is always present to a small extent as an open-chain aldehyde. By adding the Fehling reagents (Fehling (I) solution and Fehling (II) solution), the aldehyde group is oxidized to a carboxylic acid, while the Cu2+ tartrate complex is reduced to Cu+ and forms a brick red precipitate (Cu2O).
Tollens test
In the Tollens test, after addition of ammoniacal Silver nitrate, AgNO3 to the sample solution, glucose reduces Ag+ to elemental silver.Barfoed test
In Barfoed's test, a solution of dissolved copper acetate, sodium acetate and acetic acid is added to the solution of the sugar to be tested and subsequently heated in a water bath for a few minutes. Glucose and other monosaccharides rapidly produce a reddish color and reddish brown copper(I) oxide (Cu2O).Nylander's test
As a reducing sugar, glucose reacts in the Nylander's test.Other tests
Upon heating a dilute potassium hydroxide solution with glucose to 100 °C, a strong reddish browning and a caramel-like odor develops.Georg Schwedt: ''Zuckersüße Chemie''. John Wiley & Sons, 2012, , p. 102 (in German). Concentrated sulfuric acid dissolves dry glucose without blackening at room temperature forming sugar sulfuric acid. In a yeast solution, alcoholic fermentation produces carbon dioxide in the ratio of 2.0454 molecules of glucose to one molecule of Carbon dioxide, CO2. Glucose forms a black mass with stannous chloride. In an ammoniacal silver solution, glucose (as well as lactose and dextrin) leads to the deposition of silver. In an ammoniacal lead acetate solution, white lead glycoside is formed in the presence of glucose, which becomes less soluble on cooking and turns brown. In an ammoniacal copper solution, yellow copper oxide hydrate is formed with glucose at room temperature, while red copper oxide is formed during boiling (same with dextrin, except for with an ammoniacal copper acetate solution). With Picric acid, Hager's reagent, glucose forms mercury oxide during boiling. An alkaline bismuth solution is used to precipitate elemental, black-brown bismuth with glucose. Glucose boiled in an ammonium molybdate solution turns the solution blue. A solution with indigo carmine and sodium carbonate destains when boiled with glucose.Instrumental quantification
Refractometry and polarimetry
In concentrated solutions of glucose with a low proportion of other carbohydrates, its concentration can be determined with a polarimeter. For sugar mixtures, the concentration can be determined with a refractometer, for example in the Oechsle scale, Oechsle determination in the course of the production of wine.Photometric enzymatic methods in solution
The enzyme glucose oxidase (GOx) converts glucose into gluconic acid and hydrogen peroxide while consuming oxygen. Another enzyme, peroxidase, catalyzes a chromogenic reaction (Trinder reaction) of phenol with 4-Aminoantipyrine, 4-aminoantipyrine to a purple dye.Photometric test-strip method
The test-strip method employs the above-mentioned enzymatic conversion of glucose to gluconic acid to form hydrogen peroxide. The reagents are immobilised on a polymer matrix, the so-called test strip, which assumes a more or less intense color. This can be measured reflectometrically at 510 nm with the aid of an LED-based handheld photometer. This allows routine blood sugar determination by nonscientists. In addition to the reaction of phenol with 4-aminoantipyrine, new chromogenic reactions have been developed that allow photometry at higher wavelengths (550 nm, 750 nm).Amperometric glucose sensor
The electroanalysis of glucose is also based on the enzymatic reaction mentioned above. The produced hydrogen peroxide can be amperometrically quantified by anodic oxidation at a potential of 600 mV. The GOx is immobilized on the electrode surface or in a membrane placed close to the electrode. Precious metals such as platinum or gold are used in electrodes, as well as carbon nanotube electrodes, which e.g. are doped with boron. Cu–CuO nanowires are also used as enzyme-free amperometric electrodes, reaching a detection limit of 50 μmol/L. A particularly promising method is the so-called "enzyme wiring", where the electron flowing during the oxidation is transferred via a molecular wire directly from the enzyme to the electrode.Other sensory methods
There are a variety of other chemical sensors for measuring glucose. Given the importance of glucose analysis in the life sciences, numerous optical probes have also been developed for saccharides based on the use of boronic acids, which are particularly useful for intracellular sensory applications where other (optical) methods are not or only conditionally usable. In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups of sugars, there are also other probe concepts classified by functional mechanisms which use selective glucose-binding proteins (e.g. concanavalin A) as a receptor. Furthermore, methods were developed which indirectly detect the glucose concentration via the concentration of metabolized products, e.g. by the consumption of oxygen using fluorescence-optical sensors. Finally, there are enzyme-based concepts that use the intrinsic absorbance or fluorescence of (fluorescence-labeled) enzymes as reporters.Copper iodometry
Glucose can be quantified by copper iodometry.Chromatographic methods
In particular, for the analysis of complex mixtures containing glucose, e.g. in honey, chromatographic methods such as high performance liquid chromatography and gas chromatography are often used in combination with mass spectrometry. Taking into account the isotope ratios, it is also possible to reliably detect honey adulteration by added sugars with these methods. Derivatization using silylation reagents is commonly used. Also, the proportions of di- and trisaccharides can be quantified.In vivo analysis
Glucose uptake in cells of organisms is measured with 2-deoxy-D-glucose or fluorodeoxyglucose.Donard Dwyer: ''Glucose Metabolism in the Brain.'' Academic Press, 2002, , p. XIII. (18F)fluorodeoxyglucose is used as a tracer in positron emission tomography in oncology and neurology,Gesellschaft Deutscher Chemikerwayback=20100331071121 ''Anlagen zum Positionspapier der Fachgruppe Nuklearchemie''
, February 2000. where it is by far the most commonly used diagnostic agent.
References
External links
* * {{portal bar, Chemistry, Medicine Glucose, Chemical pathology Nutrition World Health Organization essential medicines Pyranoses Glycolysis