Getter Jaani Songs on:
[Wikipedia]
[Google]
[Amazon]
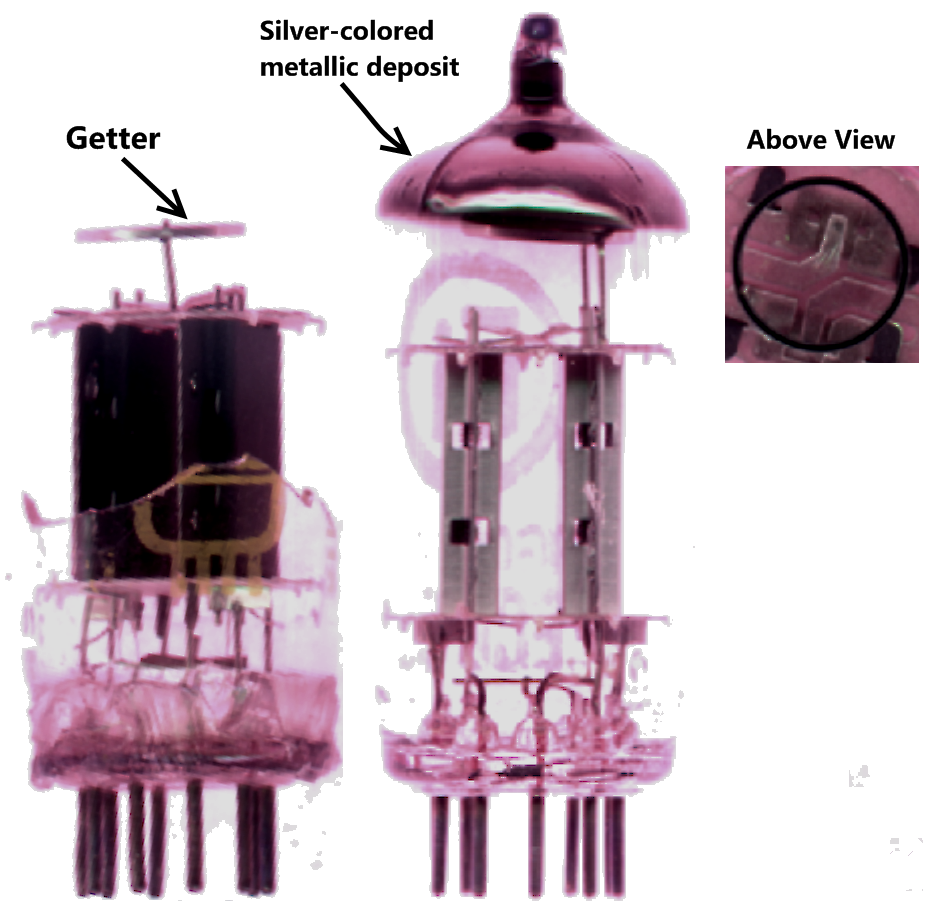 A getter is a deposit of reactive material that is placed inside a
A getter is a deposit of reactive material that is placed inside a
Tubebooks
website Different types of getter use different ways of doing this: ; Flashed getter: The getter material is held inactive in a reservoir during assembly and initial evacuation, and then heated and evaporated, usually by induction heating. The vaporized getter, usually a volatile metal, instantly reacts with any residual gas, and then condenses on the cool walls of the tube in a thin coating, the ''getter spot'' or ''getter mirror'', which continues to absorb gas. This is the most common type, used in low-power
 Flashed getters are prepared by arranging a reservoir of volatile and reactive material inside the vacuum system. After the system has been evacuated and sealed under rough vacuum, the material is heated (usually by radio frequency induction heating). After evaporating, it deposits as a coating on the interior surfaces of the system. Flashed getters (typically made with
Flashed getters are prepared by arranging a reservoir of volatile and reactive material inside the vacuum system. After the system has been evacuated and sealed under rough vacuum, the material is heated (usually by radio frequency induction heating). After evaporating, it deposits as a coating on the interior surfaces of the system. Flashed getters (typically made with
High-Q MEMS gyroscope
/ref> It is, of course, important not to heat the getter when the system is not already in a good vacuum.
How to activate getter in GU74B / 4CX800A
An Ultrahigh Vacuum Packaging Process Demonstrating Over 2 Million Q-Factor in MEMS Vibratory Gyroscopes
IEEE Sensors Letters {{Thermionic valves Vacuum tubes
 A getter is a deposit of reactive material that is placed inside a
A getter is a deposit of reactive material that is placed inside a vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often dis ...
system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by absorption. Thus the getter removes small amounts of gas from the evacuated space. The getter is usually a coating applied to a surface within the evacuated chamber.
A vacuum is initially created by connecting a container to a vacuum pump
A vacuum pump is a device that draws gas molecules from a sealed volume in order to leave behind a partial vacuum. The job of a vacuum pump is to generate a relative vacuum within a capacity. The first vacuum pump was invented in 1650 by Otto v ...
. After achieving a sufficient vacuum, the container can be sealed, or the vacuum pump can be left running. Getters are especially important in sealed systems, such as vacuum tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied.
The type kn ...
s, including cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), pictu ...
s (CRTs), Vacuum Insulating Glass (or Vacuum Glass) and vacuum insulated panel
A vacuum insulated panel (VIP) is a form of thermal insulation consisting of a gas-tight enclosure surrounding a rigid core, from which the air has been evacuated. It is used in building construction, refrigeration units, and insulated shipping c ...
s, which must maintain a vacuum for a long time. This is because the inner surfaces of the container release adsorbed gases for a long time after the vacuum is established. The getter continually removes residues of a reactive gas, such as oxygen, as long as it is desorbed from a surface, or continuously penetrating in the system (tiny leaks or diffusion through a permeable material). Even in systems which are continually evacuated by a vacuum pump, getters are also used to remove residual gas, often to achieve a higher vacuum than the pump could achieve alone. Although it is often present in minute amounts and has no moving parts, a getter behaves in itself as a vacuum pump. It is an ultimate chemical sink for reactive gases.
Getters cannot react with inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
es, though some getters will adsorb them in a reversible way. Also, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
is usually handled by adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
rather than by reaction.
Types
To avoid being contaminated by the atmosphere, the getter must be introduced into the vacuum system in an inactive form during assembly, and activated after evacuation. This is usually done by heat. on Pete Miller'Tubebooks
website Different types of getter use different ways of doing this: ; Flashed getter: The getter material is held inactive in a reservoir during assembly and initial evacuation, and then heated and evaporated, usually by induction heating. The vaporized getter, usually a volatile metal, instantly reacts with any residual gas, and then condenses on the cool walls of the tube in a thin coating, the ''getter spot'' or ''getter mirror'', which continues to absorb gas. This is the most common type, used in low-power
vacuum tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied.
The type kn ...
s.
; Non-evaporable getter (NEG): The getter remains in solid form.
Flashed getters
barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
) are commonly used in vacuum tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied.
The type kn ...
s. Most getters can be seen as a silvery metallic spot on the inside of the tube's glass envelope. Large transmission tubes and specialty systems often use more exotic getters, including aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
, strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
, caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
, and phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
.
If the getter is exposed to atmospheric air (for example, if the tube breaks or develops a leak), it turns white and becomes useless. For this reason, flashed getters are only used in sealed systems. A functioning phosphorus getter looks very much like an oxidised metal getter, although it has an iridescent
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear to gradually change color as the angle of view or the angle of illumination changes. Examples of iridescence include soap bubbles, feathers, butterfl ...
pink or orange appearance which oxidised metal getters lack. Phosphorus was frequently used before metallic getters were developed.
In systems which need to be opened to air for maintenance, a titanium sublimation pump
A titanium sublimation pump (TSP) is a type of vacuum pump used to remove residual gas in ultra-high vacuum systems, maintaining the vacuum.
Principle of operation
Its construction and principle of operation is simple. It consists of a titanium ...
provides similar functionality to flashed getters, but can be flashed repeatedly. Alternatively, nonevaporable getters may be used.
Those unfamiliar with sealed vacuum devices, such as vacuum tubes
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied.
The type known as a ...
/thermionic valves, high-pressure sodium lamp
A sodium-vapor lamp is a gas-discharge lamp that uses sodium in an excited state to produce light at a characteristic wavelength near 589 nm.
Two varieties of such lamps exist: low pressure and high pressure. Low-pressure sodium lamps are ...
s or some types of metal-halide lamps, often notice the shiny flash getter deposit and mistakenly think it is a sign of failure or degradation of the device. Contemporary high-intensity discharge lamps tend to use non-evaporable getters rather than flash getters.
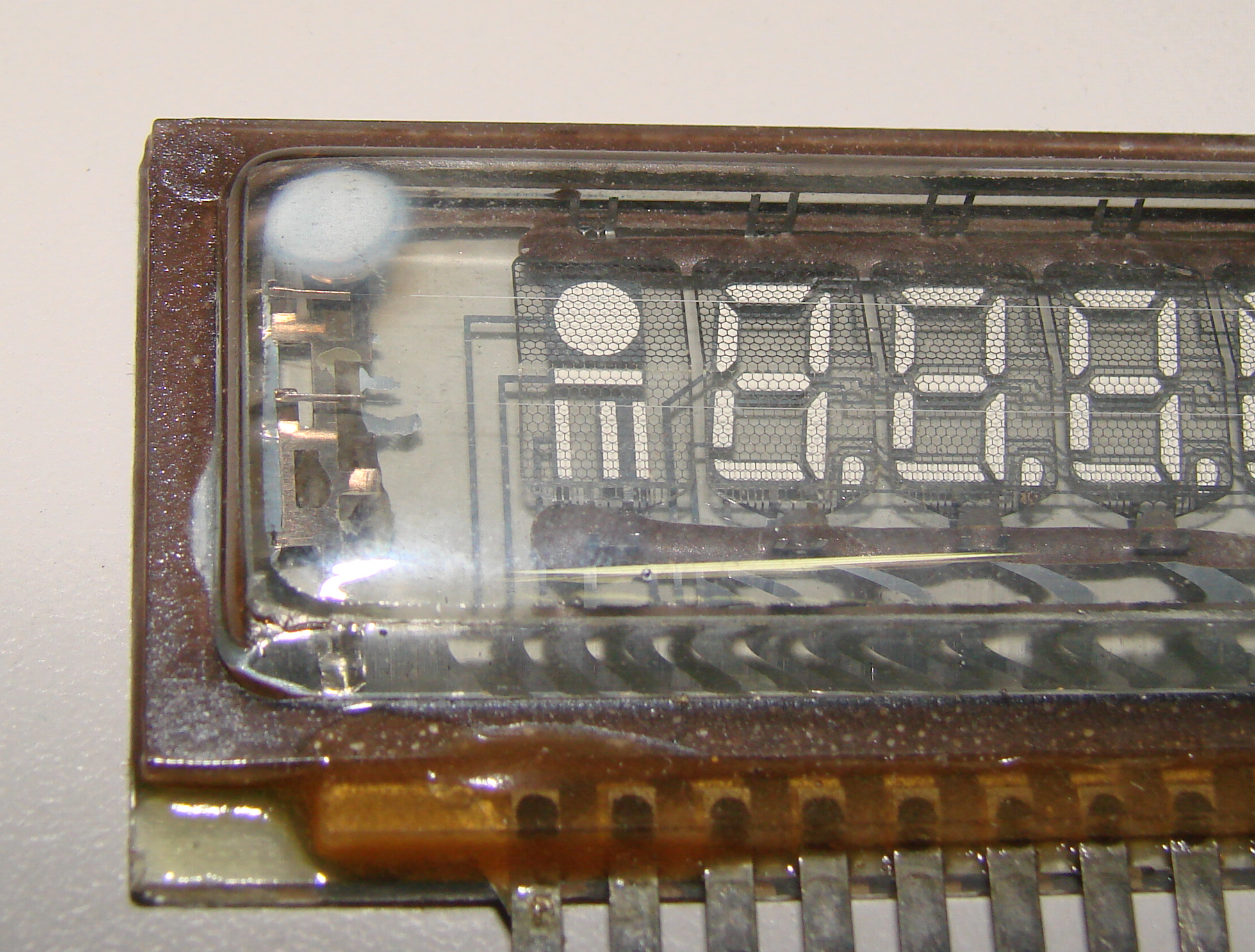
Those familiar with such devices can often make qualitative assessments as to the hardness or quality of the vacuum within by the appearance of the flash getter deposit, with a shiny deposit indicating a good vacuum. As the getter is used up, the deposit often becomes thin and translucent, particularly at the edges. It can take on a brownish-red semi-translucent appearance, which indicates poor seals or extensive use of the device at elevated temperatures. A white deposit, usually barium oxide, indicates total failure of the seal on the vacuum system, as shown in the fluorescent display module depicted above.
Activation
The typical flashed getter used in small vacuum tubes ''(seen in 12AX7 tube, top)'' consists of a ring-shaped structure made from a long strip of nickel, which is folded into a long, narrow trough, filled with a mixture ofbarium azide
Barium azide is an inorganic azide with the formula . It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.
Preparation
Barium azide may be prepared by reacting sodiu ...
and powdered glass, and then folded into the closed ring shape. The getter is attached with its trough opening facing upward toward the glass, in the specific case depicted above.
During activation, while the bulb is still connected to the pump, an RF induction heating coil connected to a powerful RF oscillator operating in the 27 MHz or 40.68 MHz ISM band is positioned around the bulb in the plane of the ring. The coil acts as the primary of a transformer and the ring as a single shorted turn. Large RF currents flow in the ring, heating it. The coil is moved along the axis of the bulb so as not to overheat and melt the ring. As the ring is heated, the barium azide decomposes into barium vapor and nitrogen. The nitrogen is pumped out and the barium condenses on the bulb above the plane of the ring forming a mirror-like deposit with a large surface area. The powdered glass in the ring melts and entraps any particles which could otherwise escape loose inside the bulb causing later problems. The barium combines with any free gas when activated and continues to act after the bulb is sealed off from the pump. During use, the internal electrodes and other parts of the tube get hot. This can cause adsorbed gases to be released from metallic parts, such as anodes (plates), grids, or non-metallic porous parts, such as sintered ceramic parts. The gas is trapped on the large area of reactive barium on the bulb wall and removed from the tube.
Non-evaporable getters
''Non-evaporable getters'', which work at high temperature, generally consist of a film of a special alloy, often primarilyzirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', ...
; the requirement is that the alloy materials must form a passivation layer at room temperature which disappears when heated.
Common alloys have names of the form St (Stabil) followed by a number:
*St 707 is 70% zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', ...
, 24.6% vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
, and the balance iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
.
*St 787 is 80.8% zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', ...
, 14.2% cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
, and the balance mischmetal.
*St 101 is 84% zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', ...
and 16% aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
.
In tubes used in electronics, the getter material coats plates within the tube which are heated in normal operation; when getters are used within more general vacuum systems, such as in semiconductor manufacturing, they are introduced as separate pieces of equipment in the vacuum chamber, and turned on when needed.
Deposited and patterned getter material is being used in microelectronics packaging to provide an ultra-high vacuum in a sealed cavity. To enhance the getter pumping capacity, the activation temperature must be maximized, considering the process limitations./ref> It is, of course, important not to heat the getter when the system is not already in a good vacuum.
See also
*Ion pump (physics)
An ion pump (also referred to as a sputter ion pump) is a type of vacuum pump which operates by sputtering a metal getter. Under ideal conditions, ion pumps are capable of reaching pressures as low as 10−11 mbar. An ion pump first ionizes ga ...
References
*Stokes, John W. ''70 Years of Radio Tubes and Valves: A Guide for Engineers, Historians, and Collectors.'' Vestal Press, 1982. *Reich, Herbert J. ''Principles of Electron Tubes. Understanding and Designing Simple Circuits.'' Audio Amateur Radio Publication, May 1995. (Reprint of 1941 original).External links
How to activate getter in GU74B / 4CX800A
An Ultrahigh Vacuum Packaging Process Demonstrating Over 2 Million Q-Factor in MEMS Vibratory Gyroscopes
IEEE Sensors Letters {{Thermionic valves Vacuum tubes