G-Strophanthin on:
[Wikipedia]
[Google]
[Amazon]
 Ouabain or (from Somali ''waabaayo'', "arrow poison" through French ''ouabaïo'') also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an
Ouabain or (from Somali ''waabaayo'', "arrow poison" through French ''ouabaïo'') also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an
 The African crested rat (''Lophiomys imhausi'') has a broad, white-bordered strip of hairs covering an area of glandular skin on the flank. When the animal is threatened or excited, the mane on its back erects and this flank strip parts, exposing the glandular area. The hairs in this flank area are highly specialised; at the tips they are like ordinary hairs, but are otherwise spongy, fibrous, and absorbent. The rat is known to deliberately chew the roots and bark of the Poison-arrow tree (''
The African crested rat (''Lophiomys imhausi'') has a broad, white-bordered strip of hairs covering an area of glandular skin on the flank. When the animal is threatened or excited, the mane on its back erects and this flank strip parts, exposing the glandular area. The hairs in this flank area are highly specialised; at the tips they are like ordinary hairs, but are otherwise spongy, fibrous, and absorbent. The rat is known to deliberately chew the roots and bark of the Poison-arrow tree (''
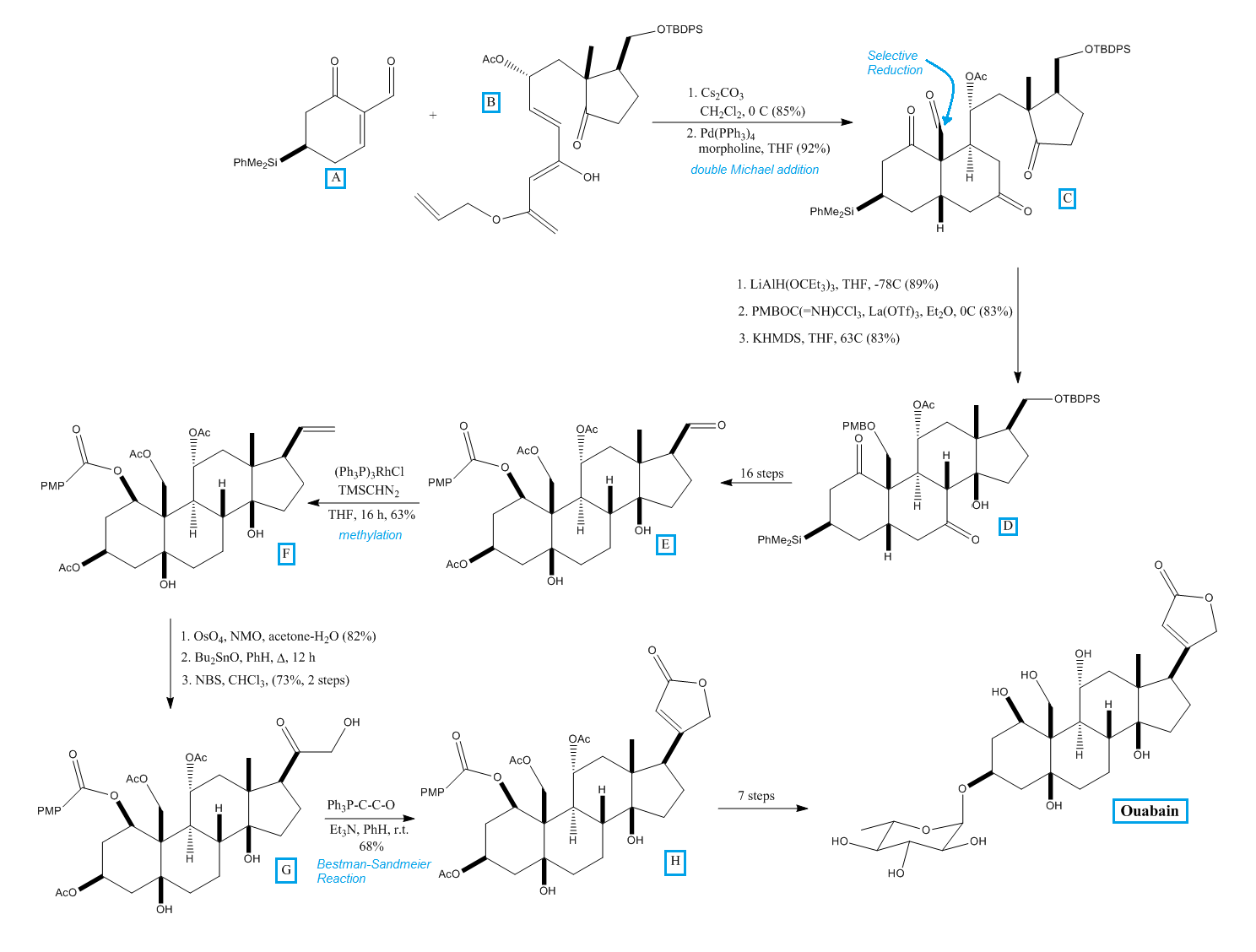 In their synthesis, Zhang ''et al.'' from the Deslongchamps laboratory condensed cyclohexenone A with Nazarov substitute B in a double Michael addition to produce tricycle C. At the indicated position, C was reduced to the aldehyde and the alcohol group was protected with p-methoxybenzyl ether (PMB) to form the aldol precursor needed to produce D. After several steps, intermediate E was produced. E contained all the required functionalities and stereochemistry needed to produce ouabain. The structure of E was confirmed by comparison against the degradation product of ouabain. Methylation of E, catalyzed by rhodium, produced F. The dehydroxylation and selective oxidation of the secondary hydroxy group of F produced G. G reacted with triphenyl phosphoranylidene ketene and the ester bonds in G were hydrolyzed to produce ouabagenin, a precursor to ouabain. The
In their synthesis, Zhang ''et al.'' from the Deslongchamps laboratory condensed cyclohexenone A with Nazarov substitute B in a double Michael addition to produce tricycle C. At the indicated position, C was reduced to the aldehyde and the alcohol group was protected with p-methoxybenzyl ether (PMB) to form the aldol precursor needed to produce D. After several steps, intermediate E was produced. E contained all the required functionalities and stereochemistry needed to produce ouabain. The structure of E was confirmed by comparison against the degradation product of ouabain. Methylation of E, catalyzed by rhodium, produced F. The dehydroxylation and selective oxidation of the secondary hydroxy group of F produced G. G reacted with triphenyl phosphoranylidene ketene and the ester bonds in G were hydrolyzed to produce ouabagenin, a precursor to ouabain. The
 Ouabain or (from Somali ''waabaayo'', "arrow poison" through French ''ouabaïo'') also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an
Ouabain or (from Somali ''waabaayo'', "arrow poison" through French ''ouabaïo'') also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an arrow poison
Arrow poisons are used to poison arrow heads or darts for the purposes of hunting and warfare. They have been used by indigenous peoples worldwide and are still in use in areas of South America, Africa and Asia. Notable examples are the poisons se ...
in eastern Africa for both hunting and warfare. Ouabain is a cardiac glycoside
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses are as treatments for co ...
and in lower doses, can be used medically to treat hypotension and some arrhythmias. It acts by inhibiting the Na/K-ATPase, also known as the sodium-potassium ion pump. However, adaptations to the alpha-subunit of the Na+/K+-ATPase
NA, N.A., Na, nA or n/a may refer to:
Chemistry and physics
* Sodium, symbol Na, a chemical element
* Avogadro constant (''N''A)
* Nucleophilic addition, a type of reaction in organic chemistry
* Numerical aperture, a number that characterizes ...
via amino acid substitutions, have been observed in certain species, namely some herbivore- insect species, that have resulted in toxin resistance.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act
The Emergency Planning and Community Right-to-Know Act of 1986 is a United States federal law passed by the 99th United States Congress located at Title 42, Chapter 116 of the U.S. Code, concerned with emergency response preparedness.
On October ...
(42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
Sources
Ouabain can be found in the roots, stems, leaves, and seeds of the ''Acokanthera schimperi
''Acokanthera schimperi'', arrow poison tree, belonging to the family Apocynaceae, is a small tree native to eastern and central Africa as well as to Yemen.
Uses
The bark, wood and roots of ''Acokanthera schimperi'' are used as an important in ...
'' and ''Strophanthus gratus
''Strophanthus gratus'' is a plant in the dogbane family Apocynaceae.
Description
''Strophanthus gratus'' is a woody liana that can grow up to , with a trunk diameter of up to . Its fragrant flowers feature a white corolla, topped by red or pur ...
'' plants, both of which are native to eastern Africa.Neuwinger, Hans Dieter. African Ethanobotany: Poisons And Drugs. Weinheim: Chapman & Hall, 1996. Print.
Mechanism of action
Ouabain is a cardiac glycoside that acts by inhibiting the Na+/K+–ATPase sodium-potassium ion pump (but it is not selective).Pubchem.ncbi.nlm.nih.gov,. 'Ouabain , C29H44O12 - Pubchem'. N.p., 2015. Web. 8 Apr. 2015. Once ouabain binds to this enzyme, the enzyme ceases to function, leading to an increase of intracellular sodium. This increase in intracellular sodium reduces the activity of thesodium-calcium exchanger
The sodium-calcium exchanger (often denoted Na+/Ca2+ exchanger, exchange protein, or NCX) is an antiporter membrane protein that removes calcium from cells. It uses the energy that is stored in the electrochemical gradient of sodium (Na+) by al ...
(NCX), which pumps one calcium ion out of the cell and three sodium ions into the cell down their concentration gradient. Therefore, the decrease in the concentration gradient of sodium into the cell which occurs when the Na/K-ATPase is inhibited reduces the ability of the NCX to function. This in turn elevates intracellular calcium. This results in higher cardiac contractility and an increase in cardiac vagal tone Vagal tone is activity of the vagus nerve, the 10th cranial nerve and a fundamental component of the parasympathetic branch of the autonomic nervous system. This branch of the nervous system is not under conscious control and is largely responsible ...
. The change in ionic gradients caused by ouabain can also affect the membrane voltage of the cell and result in cardiac arrhythmias.
Symptoms
An overdose of ouabain can be detected by the presence of the following symptoms: rapid twitching of the neck and chest musculature, respiratory distress, increased and irregular heartbeat, rise in blood pressure, convulsions, wheezing, clicking, and gasping rattling. Death is caused by cardiac arrest.Toxicology
Ouabain is a highly toxic compound with a LD50 of 5 mg/kg when administered orally to rodents.Guidechem.com,. 'Ouabain (Cas 630-60-4) Msds'. N.p., 2015. Web. 8 Apr. 2015. However, ouabain has a low bioavailability and is absorbed poorly from the alimentary tract as so much of the oral dose is destroyed. Intravenous administration results in greater available concentrations and has been shown to decrease the LD50 to 2.2 mg/kg, also in rodents. After intravenous administration, the onset of action occurs within 2–10 minutes in humans with the maximum effect enduring for 1.5 hours. Ouabain is eliminated by renal excretion, largely unchanged.Biological effects
Endogenous ouabain
In 1991, a specific high affinity sodium pump inhibitor indistinguishable from ouabain was first discovered in the human circulation and proposed as one of the potential mediators of long term blood pressure and the enhanced salt excretion following salt and volume loading. This agent was an inhibitor of the sodium pump that acted similarly todigitalis
''Digitalis'' ( or ) is a genus of about 20 species of herbaceous perennial plants, shrubs, and biennials, commonly called foxgloves.
''Digitalis'' is native to Europe, western Asia, and northwestern Africa. The flowers are tubular in sha ...
. A number of analytical techniques led to the conclusion that this circulating molecule was ouabain and that humans were producing it as an endogenous hormone. A large portion of the scientific community agreed that this inhibitor was endogenous ouabain and that there was strong evidence to indicate that it was synthesized in the adrenal gland
The adrenal glands (also known as suprarenal glands) are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which ...
. One early speculative interpretation of the analytical data led to the proposal that endogenous ouabain may have been the 11 epimer, i.e., an isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
of plant ouabain. However, this possibility was excluded by various methods including the synthesis of the 11 epimer and the demonstration that it has different chromatographic behavior from ouabain. Critically, the primary observations concerning the identification of ouabain in mammals were repeated and confirmed using a variety of tissue sources on three different continents with advanced analytical methods as summarized elsewhere
Despite widespread analytical confirmation, some questioned whether or not this endogenous substance is ouabain. The arguments were based less upon rigorous analytical data but more on the fact that immunoassays are neither entirely specific nor reliable. Hence, it was suggested that some assays for endogenous ouabain detected other compounds or failed to detect ouabain at all. Additionally, it was suggested that rhamnose, the L-sugar component of ouabain, could not be synthesized within the body despite published data to the contrary. Yet another argument against the existence of endogenous ouabain was the lack of effect of rostafuroxin
Rostafuroxin is a digitoxigenin analog that has been shown to lower blood pressure in an animal model of hypertension. It modulates the effects of the enzyme Na+/K+-ATPase, which maintains sodium and potassium ion gradients across plasma membrane ...
(a first generation ouabain receptor antagonist) on blood pressure in an unselected population of hypertensive patients.
Medical uses
Although ouabain is no longer approved for use in the USA, in France and Germany, intravenous ouabain has a long history in the treatment of heart failure, and some continue to advocate its use intravenously and orally inangina pectoris
Angina, also known as angina pectoris, is chest pain or pressure, usually caused by insufficient blood flow to the heart muscle (myocardium). It is most commonly a symptom of coronary artery disease.
Angina is typically the result of obstru ...
and myocardial infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may ...
despite its poor and variable absorption. The positive properties of ouabain regarding the prophylaxis and treatment of these two indications are documented by several studies.
Animal use of ouabain
 The African crested rat (''Lophiomys imhausi'') has a broad, white-bordered strip of hairs covering an area of glandular skin on the flank. When the animal is threatened or excited, the mane on its back erects and this flank strip parts, exposing the glandular area. The hairs in this flank area are highly specialised; at the tips they are like ordinary hairs, but are otherwise spongy, fibrous, and absorbent. The rat is known to deliberately chew the roots and bark of the Poison-arrow tree (''
The African crested rat (''Lophiomys imhausi'') has a broad, white-bordered strip of hairs covering an area of glandular skin on the flank. When the animal is threatened or excited, the mane on its back erects and this flank strip parts, exposing the glandular area. The hairs in this flank area are highly specialised; at the tips they are like ordinary hairs, but are otherwise spongy, fibrous, and absorbent. The rat is known to deliberately chew the roots and bark of the Poison-arrow tree (''Acokanthera schimperi
''Acokanthera schimperi'', arrow poison tree, belonging to the family Apocynaceae, is a small tree native to eastern and central Africa as well as to Yemen.
Uses
The bark, wood and roots of ''Acokanthera schimperi'' are used as an important in ...
''), which contains ouabain. After the rat has chewed the tree, instead of swallowing the poison it slathers the resulting masticate onto its specialised flank hairs which are adapted to absorb the poisonous mixture. It thereby creates a defense mechanism that can sicken or even kill predators which attempt to bite it.
Synthesis
The total synthesis of ouabain was achieved in 2008 by Deslongchamps laboratory in Canada.Zhang, Hongxing et al. 'Total Synthesis Of Ouabagenin And Ouabain'. Angew. Chem. 120.7 (2008): 1292-1295. Web. It was synthesized under the hypothesis that a polyanionic cyclization (doubleMichael addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
followed by aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to ...
) would allow access to a tetracyclic intermediate with the desired functionality. The figure below shows the key steps in the synthesis of ouabain.
 In their synthesis, Zhang ''et al.'' from the Deslongchamps laboratory condensed cyclohexenone A with Nazarov substitute B in a double Michael addition to produce tricycle C. At the indicated position, C was reduced to the aldehyde and the alcohol group was protected with p-methoxybenzyl ether (PMB) to form the aldol precursor needed to produce D. After several steps, intermediate E was produced. E contained all the required functionalities and stereochemistry needed to produce ouabain. The structure of E was confirmed by comparison against the degradation product of ouabain. Methylation of E, catalyzed by rhodium, produced F. The dehydroxylation and selective oxidation of the secondary hydroxy group of F produced G. G reacted with triphenyl phosphoranylidene ketene and the ester bonds in G were hydrolyzed to produce ouabagenin, a precursor to ouabain. The
In their synthesis, Zhang ''et al.'' from the Deslongchamps laboratory condensed cyclohexenone A with Nazarov substitute B in a double Michael addition to produce tricycle C. At the indicated position, C was reduced to the aldehyde and the alcohol group was protected with p-methoxybenzyl ether (PMB) to form the aldol precursor needed to produce D. After several steps, intermediate E was produced. E contained all the required functionalities and stereochemistry needed to produce ouabain. The structure of E was confirmed by comparison against the degradation product of ouabain. Methylation of E, catalyzed by rhodium, produced F. The dehydroxylation and selective oxidation of the secondary hydroxy group of F produced G. G reacted with triphenyl phosphoranylidene ketene and the ester bonds in G were hydrolyzed to produce ouabagenin, a precursor to ouabain. The glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
of ouabagenin with rhamnose
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most o ...
produced ouabain.
History
Africa
Poisons derived from ''Acokanthera'' plants are known to have been used in Africa as far back as the 3rd century BC whenTheophrastus
Theophrastus (; grc-gre, Θεόφραστος ; c. 371c. 287 BC), a Greek philosopher and the successor to Aristotle in the Peripatetic school. He was a native of Eresos in Lesbos.Gavin Hardy and Laurence Totelin, ''Ancient Botany'', Routledge ...
reported a toxic substance that the Ethiopians would smear on their arrows. The poisons derived from this genus of plants were used throughout eastern Africa, typically as arrow poison
Arrow poisons are used to poison arrow heads or darts for the purposes of hunting and warfare. They have been used by indigenous peoples worldwide and are still in use in areas of South America, Africa and Asia. Notable examples are the poisons se ...
s for hunting and warfare. ''Acokanthera schimperi'', in particular, exhibits a very large amount of ouabain, which the Kenyans, Tanzanians, Rwandans, Ethiopians, and Somalis would use as an arrow poison.
The poison was extracted from the branches and leaves of the plant by boiling them over a fire. Arrows would then be dipped into the concentrated black tar-like juice that formed. Often, certain magical additives were also mixed in with the ouabain extract in order to make the poison work according to the hunter's wishes. In Kenya, the Giriama
The Giriama (also called Giryama) are one of the nine ethnic groups that make up the Mijikenda (which literally translates to "nine towns").
The Mijikenda occupy the coastal strip extending from Lamu in the north to the Kenya/Tanzania border in ...
and Langulu poison makers would add an elephant shrew to the poison mixture in order to facilitate the pursuit of their prey. They had observed that an elephant shrew would always run straight ahead or follow a direct path and thought that these properties would be transferred to the poison. A poisonous arrow made with this shrew was thought to cause the hunted animal to behave like the shrew and run in a straight path. In Rwanda members of the Nyambo tribe, also known poison arrow makers, harvest the ''Aconkathera'' plants according to how many dead insects are found under it - more dead insects under a shrub indicating a higher potency of poison.
Although ouabain was used as an arrow poison primarily for hunting, it was also used during battle. One example of this occurred during a battle against the Portuguese, who had stormed Mombasa in 1505. Portuguese records indicated that they had suffered a great deal from the poisoned arrows.
Europe
European imperial expansion and exploration into Africa overlapped with the rise of the Europeanpharmaceutical industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, vaccinate them, or alleviate symptoms. ...
towards the end of the nineteenth century.Osseo-Asare, A. D. 'Bioprospecting And Resistance: Transforming Poisoned Arrows Into Strophantin Pills In Colonial Gold Coast, 1885-1922'. Social History of Medicine 21.2 (2008): 269-290. Web. British troops were the target of arrows poisoned with the extracts of various ''Strophanthus'' species. They were familiar with the deadly properties of these plants and brought samples back to Europe. Around this time, interest in the plant grew. It was known that ouabain was a cardiac poison, but there was some speculation about its potential medical uses.
In 1882, ouabain was first isolated from the plant by the French chemist Léon-Albert Arnaud as an amorphous substance, which he identified as a glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. ...
. Ouabain was seen as a possible treatment for certain cardiac conditions.
See also
*Strophanthidin
k-Strophanthidin is a cardenolide found in species of the genus ''Strophanthus''. It is the aglycone of k-strophanthin, an analogue of ouabain. k-strophanthin is found in the ripe seeds of Strophanthus kombé and in the lily Convallaria.
K-St ...
References
External links
* * * * {{Cardiac glycosides Cardenolides Pyranoses Cyclohexanols Cyclopentanols Primary alcohols Tertiary alcohols Total synthesis ATPase inhibitors