Fétizon Oxidation on:
[Wikipedia]
[Google]
[Amazon]
Fétizon oxidation is the
 The rate limiting step of this reaction is proposed to be the initial association of the alcohol to the silver ions. As a result, the presence of even weakly associating ligands to the silver can inhibit the reaction greatly. As a result, even slightly polar solvents of any variety, such as
The rate limiting step of this reaction is proposed to be the initial association of the alcohol to the silver ions. As a result, the presence of even weakly associating ligands to the silver can inhibit the reaction greatly. As a result, even slightly polar solvents of any variety, such as  Increasing the amount of celite used in the reagent accelerates the rate of the reaction by increasing the surface area available to react. However, increasing the amount of celite past 900 grams per mole of silver(I) carbonate begins to slow the reaction due to dilution effects.
Increasing the amount of celite used in the reagent accelerates the rate of the reaction by increasing the surface area available to react. However, increasing the amount of celite past 900 grams per mole of silver(I) carbonate begins to slow the reaction due to dilution effects.
 The mildness and structural sensitivity of the reagent also makes this reagent ideal for the monooxidation of a symmetric diol.
The mildness and structural sensitivity of the reagent also makes this reagent ideal for the monooxidation of a symmetric diol.
 Lactols are extremely sensitive to Fétizon's reagent, being oxidized very quickly to a lactone functionality. This allows for the selective oxidation of lactols in the presence of other alcohols. This also allows for a classic use of Fétizon's reagent to form lactones from a primary diol. By oxidizing one of the alcohols to an aldehyde, the second alcohol equilibrates with the aldehyde to form a lactol which is reacted quickly with more Fétizon's reagent to trap the cyclic intermediate as a lactone. This method allows for the synthesis of seven-member lactones which are traditionally more challenging to synthesize.
Lactols are extremely sensitive to Fétizon's reagent, being oxidized very quickly to a lactone functionality. This allows for the selective oxidation of lactols in the presence of other alcohols. This also allows for a classic use of Fétizon's reagent to form lactones from a primary diol. By oxidizing one of the alcohols to an aldehyde, the second alcohol equilibrates with the aldehyde to form a lactol which is reacted quickly with more Fétizon's reagent to trap the cyclic intermediate as a lactone. This method allows for the synthesis of seven-member lactones which are traditionally more challenging to synthesize.




 ,3diols have a tendency to eliminate water following the monooxidation by Fétizon's reagent to form an enone.
,3diols have a tendency to eliminate water following the monooxidation by Fétizon's reagent to form an enone.
 Under differing structural conditions, ,2diols can form diketones in the presence of Fétizon's reagent. However, oxidative carbon-carbon bond cleavage may also occur.
Under differing structural conditions, ,2diols can form diketones in the presence of Fétizon's reagent. However, oxidative carbon-carbon bond cleavage may also occur.
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of primary and secondary alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s utilizing the compound silver(I) carbonate absorbed onto the surface of celite
Diatomaceous earth (), diatomite (), or kieselgur/kieselguhr is a naturally occurring, soft, siliceous sedimentary rock that can be crumbled into a fine white to off-white powder. It has a particle size ranging from more than 3 μm to les ...
also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactol
In organic chemistry, a lactol is the cyclic equivalent of a hemiacetal or a hemiketal.
The compound is formed by the intramolecular nucleophilic addition of a hydroxyl group to the carbonyl group of an aldehyde or a ketone.
A lactol is often ...
s makes the Fétizon oxidation a useful method to obtain lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s from a diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
. The reaction is inhibited significantly by polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
groups within the reaction system as well as steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
of the α-hydrogen of the alcohol.
Preparation
Fétizon's reagent is typically prepared by addingsilver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic' ...
to an aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
of a carbonate, such as sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
or potassium bicarbonate
Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.
Production and reactivity
It is manufactured by treating an ...
, while being vigorously stirred in the presence of purified celite.
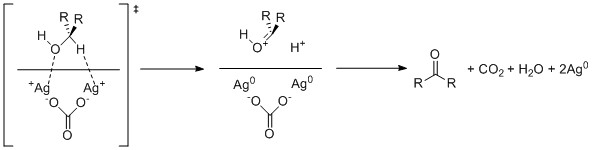
Mechanism
A proposed mechanism for the oxidation of an alcohol by Fétizon's reagent involves single electron oxidation of both the alcoholic oxygen and the hydrogen alpha to the alcohol by two atoms of silver(I) within the celite surface. Thecarbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
ion then proceeds to deprotonate the resulting carbonyl generating bicarbonate which is further protonated by the additionally generated hydrogen cation to cause elimination of water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
and generation of carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
.

ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
or methyl ethyl ketone
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nat ...
, are avoided when using this reagent as they competitively associate with the reagent. Additional polar functionalities of the reactant should also be avoided whenever possible as even the presence of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
can sometimes reduce the reactivity of a substrate 50 fold. Commonly employed solvents such as benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
and xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are sub ...
are extremely non-polar and further acceleration of the reaction can be achieved through the use of the more non-polar heptane
Heptane or ''n''-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point ...
. The solvent is also typically refluxed to drive the reaction with heat and remove the water generated by the reaction through azeotropic distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to genera ...
.
Steric hindrance of the hydrogen alpha to the alcohol is a major determination of the rate of oxidation as it effects the rate of association. Tertiary alcohols lacking an alpha hydrogen are selected against and generally do not oxidize in the presence of Fétizon's reagent.
 Increasing the amount of celite used in the reagent accelerates the rate of the reaction by increasing the surface area available to react. However, increasing the amount of celite past 900 grams per mole of silver(I) carbonate begins to slow the reaction due to dilution effects.
Increasing the amount of celite used in the reagent accelerates the rate of the reaction by increasing the surface area available to react. However, increasing the amount of celite past 900 grams per mole of silver(I) carbonate begins to slow the reaction due to dilution effects.
Reactivity
Fétizon's reagent is used primarily in the oxidation of primary or secondary alcohols toaldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s with a slight selectivity toward secondary alcohols and unsaturated alcohols. The reaction is typically done in a refluxing dry non-polar organic solvent with copious stirring. The reaction time varies with the structure of the alcohol and is typically completed within three hours. A very attractive property of Fétizon's reagent is its ability to be separated from the reaction product by physically filtering it out and washing with benzene.
The inability of Fétizon's reagent to oxidize tertiary alcohols makes it extremely useful in the monooxidation of a ,2diol in which one of the alcohols is tertiary while avoiding cleavage of the carbon-carbon bond.
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
functional groups can be oxidized to their respective quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
forms. These quinones can further couple within solution producing numerous dimerizations depending upon their substituents.
uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s have been shown to oxidize in the presence of Fétizon's reagent to enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and th ...
s and iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
cations that have been trapped, but can also be selected against in a compound with more easily oxidized alcohol functionalities.
Fétizon's reagent can also being used to facilitate cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
of a 4-hydroxy-2-furoquinilone and an olefin to form dihydrofuroquinolinones.
Protecting groups
Para-methoxybenzyl (PMB) is a commonly used protecting group for alcohols against Fétizon's reagent. As Fétizon's oxidation is a neutral reaction, acid and base sensitive protecting groups are also compatible with the reagent and by products generated.Sensitive groups
While tertiary alcohols are typically not affected by Fétizon's reagent, tertiary propargylic alcohols have been shown to oxidize under these conditions and results in the fragmentation of the alcohol with analkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
leaving group.
Halohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethan ...
s that possess a trans stereochemistry have been demonstrated to form epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
s and transposed products in the presence of Fétizon's reagent. Halohydrins possessing a cis-stereochemistry seem to perform a typical Fétizon's oxidation to a ketone.
Applications
Since its discovery as a useful method of oxidation, Fétizon's reagent has been used in the total synthesis of numerous molecules such as (±)-bukittinggine. Fétizon's reagent has also been employed extensively in the study of various sugar chemistry, to achieve selective oxidation of tri and tetra methylated aldoses to aldolactones, oxidation of D-xylose and L-arabinose to D-threose and L-erythrose respectively, and oxidation of L-sorbose to afford L-threose among many others.References
{{reflist, 35em Organic oxidation reactions Name reactions