Functionalized Polyolefins on:
[Wikipedia]
[Google]
[Amazon]
Functionalized polyolefins are olefin polymers with polar and nonpolar functionalities attached onto the polymer backbone. There has been an increased interest in functionalizing polyolefins due to their increased usage in everyday life.
 Post-functionalization of polyolefin occurs as the name suggests: functionalization occurs after a non-functionalized polyolefin is synthesized. One of the most common way to attach functionality onto a preexisting polymer backbone is through free radical reaction. Free radicals can be formed through
Post-functionalization of polyolefin occurs as the name suggests: functionalization occurs after a non-functionalized polyolefin is synthesized. One of the most common way to attach functionality onto a preexisting polymer backbone is through free radical reaction. Free radicals can be formed through
 Ring-opening metathesis polymerization (
Ring-opening metathesis polymerization (
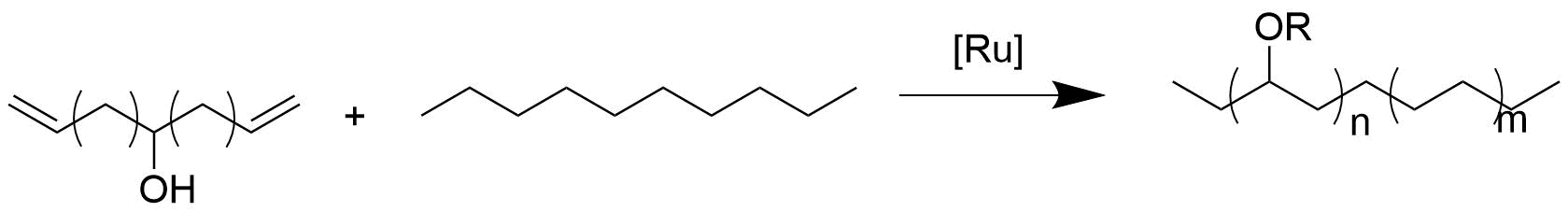

 Catalytic polymerization appears to have the most control compared to other polymerization methods for randomly functionalized polyolefins. Catalytic routes predominantly undergo a coordination/
Catalytic polymerization appears to have the most control compared to other polymerization methods for randomly functionalized polyolefins. Catalytic routes predominantly undergo a coordination/
 End functionalized polyolefins are polyolefin with functionality either at one end or at both ends of the chain. One example of end functionalization is through
End functionalized polyolefins are polyolefin with functionality either at one end or at both ends of the chain. One example of end functionalization is through

Polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
s are virtually ubiquitous in everyday life, from consumer food packaging to biomedical applications; therefore, efforts must be made to study catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
pathways towards the attachment of various functional groups onto polyolefins in order to affect the material's physical properties.
Based on the polyolefin structure, functionalized polyolefin can be categorized into four main groups: randomly functionalized polyolefins, end-functionalized polyolefins, block polyolefins, and graft polyolefins.
Randomly functionalized polyolefin
Randomly functionalized polyolefins have differing types, location, and amount of functionality on the polyolefin backbone. Randomly functionalized polyolefins can be synthesized through many familiar combination of polymerization techniques including: post-functionalization, ROMP/hydrogenation, ADMET/hydrogenation, radical polymerization, and catalytic copolymerization.Post-functionalization
 Post-functionalization of polyolefin occurs as the name suggests: functionalization occurs after a non-functionalized polyolefin is synthesized. One of the most common way to attach functionality onto a preexisting polymer backbone is through free radical reaction. Free radicals can be formed through
Post-functionalization of polyolefin occurs as the name suggests: functionalization occurs after a non-functionalized polyolefin is synthesized. One of the most common way to attach functionality onto a preexisting polymer backbone is through free radical reaction. Free radicals can be formed through plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
, peroxide initiation, etc. When there is a free radical on the polyolefin chain, maleic anhydride
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and poly ...
can be attached to promote further functionalization. Another approach is through direct insertion of carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s onto the polyolefin backbone. Though post-functionalization techniques are viable for the insertion of functional groups, harsh conditions must be used since regular non-functionalized polyolefins are highly unreactive.
Ring-opening metathesis polymerization (ROMP)
 Ring-opening metathesis polymerization (
Ring-opening metathesis polymerization (ROMP Romp or ROMP may refer to:
* IBM ROMP
The ROMP is a reduced instruction set computer (RISC) microprocessor designed by IBM in the late 1970s. It is also known as the Research OPD Miniprocessor (after the two IBM divisions that collaborated on i ...
) must occur first followed by hydrogenation of the opened product. For example, functionalized cyclooctenes can result in functionalized polyolefins via ruthenium complex catalyzed ROMP. Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
s of ethyl and vinyl acetate can be synthesized via this process. First, a cycloctene functionalized with an ester functionality at the position 5 carbon reacts with a ruthenium complex. Next, the resulting open-ringed product is treated with hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
to hydrogenate the double bond resulting in ethane and vinyl acetate copolymer.
Acyclic diene metathesis (ADMET)

Acyclic diene metathesis Acyclic diene metathesis or ADMET (distinguish from ADME) is a special type of olefin metathesis used to polymerize terminal dienes to polyenes:
The new double bonds formed can be in cis- or trans-configurations. The exact ratio depends on th ...
(ADMET) is similar to ROMP in that subsequent hydrogenation is required. ADMET requires a certain type of diene in order for the polymerization to occur. Ruthenium complexes can once again be used for ADMET polymerization. In this case, an α,ω-diene monomer with functionality is required.
Radical polymerization

Radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanis ...
occurs with an olefin and a vinyl monomer. Since olefins are not very reactive, harsh conditions must be met in order for the polymerization to occur. Ethylene and ethyl acrylate can react together to perform free radical polymerization. In this process, boron trifluoride can be used as the protected group for the ethyl acrylate.
Catalytic polymerization
 Catalytic polymerization appears to have the most control compared to other polymerization methods for randomly functionalized polyolefins. Catalytic routes predominantly undergo a coordination/
Catalytic polymerization appears to have the most control compared to other polymerization methods for randomly functionalized polyolefins. Catalytic routes predominantly undergo a coordination/migratory insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism ...
pathway. The functionality of the olefin highly affects the reactivity of the olefin, and hence its relative rate of coordination. Examples of early transition metal catalysts includes titanium and zirconium complexes. Early transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s can easily form oxides; therefore, protection groups, like the use of methylaluminoxane
Methylaluminoxane, commonly called MAO, is a mixture of organoaluminium compounds with the approximate formula (Al(CH3)O)''n''. It is usually encountered as a solution in (aromatic) solvents, commonly toluene but also xylene, cumene, or mesitylene ...
(MAO) due to its Lewis acidity
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any s ...
, can be used to prevent side reactions from happening. As a cocatalyst, MAO is well known for their use in metallocene chemistry as they activate metallocene complexes for olefin polymerization. To remove the MAO protecting group, the reaction can be treated with acid. Instead of MAO, trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is ch ...
(TMS) have also been used to protection functional groups such as amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
, since the amine functionality can easily react with other olefins to form branched polymer chains.
Another useful reaction is the use of zirconium metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
complexes to copolymerize olefin with borane monomer. After reaction with a borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
monomer, such as 9-borabicyclonoane (9-BBN
9-Borabicyclo .3.1onane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substr ...
), subsequent functionalization can result in hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
functionalities.
Turning from early transition metals to late transition metals, palladium and nickel catalysts have been used to copolymerize ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
and methylacrylate.
End functionalized polyolefin
 End functionalized polyolefins are polyolefin with functionality either at one end or at both ends of the chain. One example of end functionalization is through
End functionalized polyolefins are polyolefin with functionality either at one end or at both ends of the chain. One example of end functionalization is through living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer r ...
. Using a vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
terminated polypropene chain, subsequent reaction with carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and acid can result in an aldehyde terminated polypropene chain. This reaction moves forward under low temperature conditions (~-78 °C). Through metallocene supported polymerization, chain transfer can occur with the use of a borane chain transfer agent, which results in an end functionalized polymer chain. One advantage of this chain transfer
Chain transfer is a polymerization Chemical reaction, reaction by which the activity of a growing polymer chain is transferred to another molecule.
:P• + XR' → PX + R'•
Chain transfer reactions reduce the average molecular weight of the fi ...
process is the limited use of metals, which decreases cost.
Block and graft polyolefin

Block
Block or blocked may refer to:
Arts, entertainment and media Broadcasting
* Block programming, the result of a programming strategy in broadcasting
* W242BX, a radio station licensed to Greenville, South Carolina, United States known as ''96.3 ...
and graft
Graft or grafting may refer to:
*Graft (politics), a form of political corruption
* Graft, Netherlands, a village in the municipality of Graft-De Rijp
Science and technology
*Graft (surgery), a surgical procedure
*Grafting, the joining of plant t ...
polyolefin can provide high amount of functional groups onto the polymer chain. Synthesis of both block and graft functionalized polyolefin proceed through a combination of polymerization reactions, most notably via coordination/insertion mechanism and radical polymerization. Some disadvantages of this method is the lack of controlled polymerization and the requirement of multi-step mechanisms.
References
{{reflist Polyolefins