Fructose-2,6-bisphosphatase on:
[Wikipedia]
[Google]
[Amazon]
Phosphofructokinase-2 ( 6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of
 In
In
domain dephosphorylates Fru-2,6-P2 with the addition of water. This opposing chemical reaction is: :beta-D-fructose 2,6-bisphosphate + H2O D-fructose 6-phosphate + phosphate : Because of the enzyme's dual functions, it can be categorized into multiple families. Through categorization by the kinase reaction, this enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (
 * M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.
* M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.
 *
*

6-phosphofructokinase of Arabidopsis thaliana at genome.jp
''This article incorporates text from the public domain
' EC 2.7.1 EC 3.1.3 Glycolysis Genes on human chromosome 10 Enzymes of known structure {{InterPro content, IPR013079
glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
and gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrat ...
in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-''P''2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru ...
(Fru-2,6-P2) from substrate fructose-6-phosphate
Fructose 6-phosphate (sometimes called the Neuberg ester) is a derivative of fructose, which has been phosphorylated at the 6-hydroxy group. It is one of several possible fructosephosphates. The β-D-form of this compound is very common in cells. ...
. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
and mannose metabolism
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation ...
. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases, however, they share little sequence similarity, although a few residues
Residue may refer to:
Chemistry and biology
* An amino acid, within a peptide chain
* Crop residue, materials left after agricultural processes
* Pesticide residue, refers to the pesticides that may remain on or in food after they are appli ...
seem key to their interaction with fructose 6-phosphate.
PFK-2 is known as the "bifunctional enzyme" because of its notable structure: though both are located on one protein homodimer, its two domains act as independently functioning enzymes. One terminus serves as a kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule don ...
domain (for PFK-2) while the other terminus acts as a phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate ...
domain (FBPase-2).
In mammals, genetic mechanisms encode different PFK-2 isoforms to accommodate tissue specific needs. While general function remains the same, isoforms feature slight differences in enzymatic properties and are controlled by different methods of regulation; these differences are discussed below.
Structure
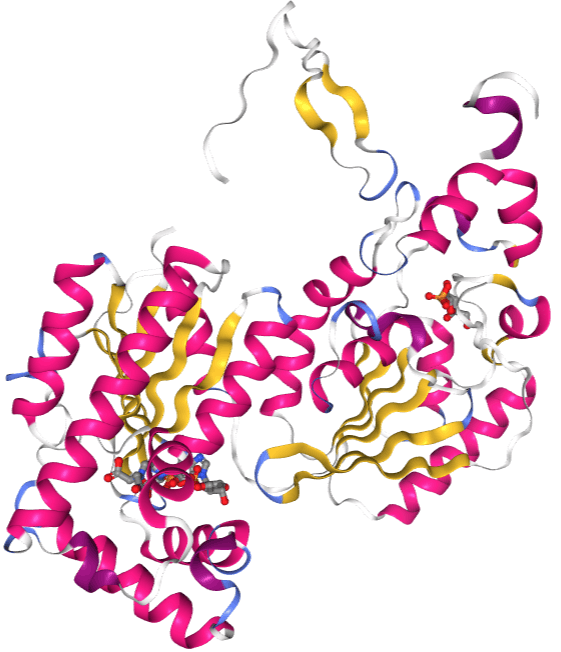
The monomers of the bifunctional protein are clearly divided into two functional domains. The kinase domain is located on the N-terminal. It consists of a central six-stranded β sheet, with five parallel strands and an antiparallel edge strand, surrounded by seven α helices. The domain contains nucleotide-binding fold (nbf) at the C-terminal end of the first β-strand. The PFK-2 domain appears to be closely related to the superfamily of mononucleotide binding proteins including adenylate cyclase. On the other hand, the phosphatase domain is located on the C-terminal. It resembles the family of proteins that include phosphoglycerate mutases and acid phosphatases. The domain has a mixed α/ β structure, with a six-stranded central β sheet, plus an additional α-helical subdomain that covers the presumed active site of the molecule. Finally, the N-terminal region modulates PFK-2 and FBPase2 activities, and stabilizes the dimer form of the enzyme. While this central catalytic core remains conserved in all forms of PFK-2, slight structural variations exist in isoforms as a result of different amino acid sequences or alternative splicing. With some minor exceptions, the size of PFK-2 enzymes is typically around 55 kDa. Researchers hypothesize that the unique bifunctional structure of this enzyme arose from a gene fusion event between a primordial bacterial PFK-1 and a primordial mutase/phosphatase.Function
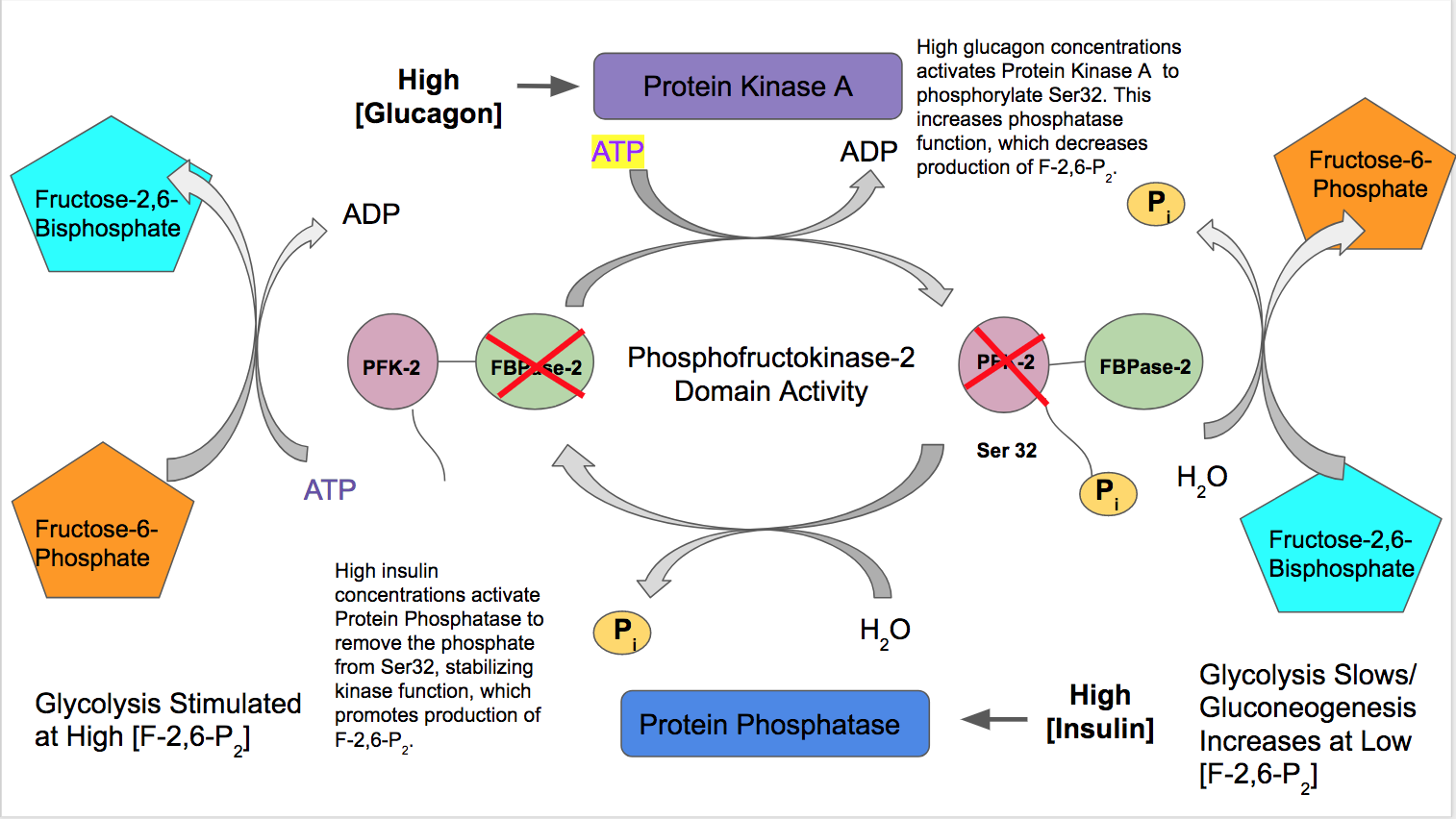
This enzyme's main function is to synthesize or degrade allosteric regulator Fru-2,6-P2 in response to glycolytic needs of the cell or organism, as depicted in the accompanying diagram. In
In enzymology
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
, a 6-phosphofructo-2-kinase () is an enzyme that catalyzes
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
the chemical reaction:
:ATP + beta-D-fructose 6-phosphate ADP + beta-D-fructose 2,6-bisphosphate
Thus, the kinase domain hydrolyzes ATP to phosphorylate the carbon-2 of fructose-6-phosphate, producing Fru-2,6-P2 and ADP. A phosphohistidine intermediate is formed within the reaction.
:At the other terminal, the fructose-2,6-bisphosphate 2-phosphatase ( ECbr>3.1.3.46domain dephosphorylates Fru-2,6-P2 with the addition of water. This opposing chemical reaction is: :beta-D-fructose 2,6-bisphosphate + H2O D-fructose 6-phosphate + phosphate : Because of the enzyme's dual functions, it can be categorized into multiple families. Through categorization by the kinase reaction, this enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (
phosphotransferase
Phosphotransferases are a category of enzymes ( EC number 2.7) that catalyze phosphorylation reactions. The general form of the reactions they catalyze is:
:A-P + B \rightleftharpoons B-P + A
Where ''P'' is a phosphate group and A and B are the do ...
s) with an alcohol group as acceptor. On the other hand, the phosphatase reaction is characteristic of the family of hydrolases, specifically those acting on phosphoric monoester bonds.
Regulation
In almost all isoforms, PFK-2 undergoes covalent modification through phosphorylation/dephosphorylation based on the cell's hormonal signaling. Phosphorylation of a specific residue may prompt a shift that stabilizes either kinase or phosphatase domain function. This regulation signal thus controls whether F-2,6-P2 will be synthesized or degraded. Furthermore, the allosteric regulation of PFK2 is very similar to the regulation ofPFK1
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes () of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of gl ...
. High levels of AMP or phosphate group signifies a low energy charge state and thus stimulates PFK2. On the other hand, a high concentration of phosphoenolpyruvate (PEP) and citrate signifies that there is a high level of biosynthetic precursor and hence inhibits PFK2. Unlike PFK1, PFK2 is not affected by ATP concentration.
#
Isozymes
Protein isozymes are enzymes that catalyze the same reaction but are encoded with different amino acid sequences and as such, display slight differences in protein characteristics. In humans, the four genes that encode phosphofructokinase 2 proteins include PFKFB-1, PFKFB2,PFKFB3
''PFKFB3'' is a gene that encodes the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 enzyme in humans. It is one of 4 tissue-specific PFKFB isoenzymes identified currently (PFKFB1-4).
Gene
The PFKFB3 gene is mapped to single locus on ch ...
and PFKFB4
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 also known as PFKFB4 is an enzyme which in humans is encoded by the ''PFKFB4'' gene.
Function
The bifunctional 6-phosphofructo-2-kinase ()/fructose-2,6-bisphosphatase () (PFKFB) regulates t ...
.
Multiple mammalian isoforms of the protein have been reported to date, difference rising by either the transcription of different enzymes or alternative splicing. While the structural core that catalyzes the PFK-2/FBPase-2 reaction is highly conserved across isoforms, the major differences arise from highly variable flanking sequences in the isoform amino and carboxyl terminals. Because these areas often contain phosphorylation sites, changes in amino acid composition or terminal length may result in vastly different enzyme kinetics and characteristics. Each variant differs in their primary tissue of expression, response to protein kinase regulation, and ratio of kinase/phosphatase domain activity. While multiple types of isozymes may consist in a tissue, isozymes are identified by their primary tissue expression and tissue of discovery below.
PFKB1: Liver, muscle, and fetal
Located on the X chromosome, this gene is the most well-known of the four genes particularly because it encodes the highly researched liver enzyme. Variable mRNA splicing of PFKB1 yields three different promoters (L, M and F) and therefore, three tissue-specific variants that differ in regulation: * L-Type: liver tissue **Insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism o ...
activates liver PFK-2 function to indicate a high abundance of blood glucose is available for glycolysis. Insulin activates a protein phosphatase which dephosphorylates the PFK-2 complex and causes favored PFK-2 activity. PFK-2 then increases production of F-2,6-P2. As this product allosterically activates PFK-1, it activates glycolysis and inhibits gluconeogenesis.
** In contrast, glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a Glucagon (medicati ...
increases FBPase-2 activity. At low blood glucose concentrations, glucagon triggers a cAMP signal cascade and in turn, Protein Kinase A (PKA) phosphorylates Serine 32 near the N-terminus. This inactivates the bifunctional enzyme's ability to act as a kinase and stabilizes the phosphatase activity. Therefore, glucagon decreases concentrations of F-2,6-P2, slows rates of glycolysis, and stimulates the gluconeogenesis pathway.
 * M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.
* M-Type: skeletal muscle tissue; F-Type: fibroblast and fetal tissue
** In contrast to most other PFK-2 tissues, PFK-2 in both skeletal muscle and fetal tissue is solely regulated by concentrations of Fructose-6-phosphate. Within their first exon, there are no regulatory sites that require phosphorylation/dephosphorylation to provoke a change in function. High concentrations of F-6-P will activate kinase function and increase rates of glycolysis, whereas low concentrations of F-6-P will stabilize phosphatase action.
PFKB2: Cardiac (H-Type)
The PFKB2 gene is located on chromosome 1. When greater concentrations of adrenaline and/or insulin hormone are circulated, a Protein Kinase A pathway is activated which phosphorylates either Serine 466 or Serine 483 in the C-terminus. Alternatively,Protein Kinase B
Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transc ...
may also phosphorylate these regulatory sites, which are part of the FBPase-2 domain. When this serine residue is phosphorylated, FBPase-2 function is inactivated and greater PFK-2 activity is stabilized.
PFKB3: Brain, placental, and inducible
PFKB3 is located on chromosome 10 and transcribes two major isoforms, inducible type and ubiquitous type. These forms differ in alternative splicing of Exon 15 in their C-terminus. However, they are similar in that for both, glucagon activates a cyclic AMP pathway; this results in Protein Kinase A, Protein Kinase C, or AMP-activated Protein Kinase phosphorylating a regulatory residue on Serine 461 in the C-terminus to stabilize PFK-2 kinase function. Furthermore, both isoforms transcribed from this gene are noted for having a particularly high, dominant rate of kinase activity as indicated by a kinase/phosphatase activity ratio of 700 (whereas the liver, heart, and testis isozymes respectively have PFK-2/FBPase-2 ratios of 1.5, 80, and 4). Therefore, PFKB3 in particular consistently produces large amounts of F-2,6-P2 and sustains high rates of glycolysis. * I-Type: Inducible ** This isoform's name is a result of its increased expression in response to hypoxic stress; its formation isinduced
Induce may refer to:
* Induced consumption
* Induced innovation
* Induced character
* Induced coma
* Induced menopause
* Induced metric
* Induced path
* Induced topology
* Induce (musician), American musician
See also
* Inducement (disambiguation ...
by lack of oxygen. This type is highly expressed in rapidly proliferating cells, especially tumor cells.
* U-Type: Ubiquitous; also known as placental or brain
**
** Though discovered separately in the placental, pancreatic-β-islet, or brain tissues, the various isoforms appear identical. The tissues it was discovered in all require great energy to function, which may explain PFKB3's advantage of such high kinase-phosphatase activity ratio.
** The brain isoform in particular has lengthy N- and C-terminus regions such that this type is almost twice as large as the typical PFK-2, at around 110 kDa.
 *
*
PFKB4: Testis (T-Type)
Gene PFKB4, located on chromosome 3, expresses PFK-2 in human testis tissue. PFK-2 enzymes encoded by PFK-4 are comparable to the liver enzyme in size at around 54kDa, and like the muscle tissue, do not contain a protein kinase phosphorylation site. While less research has clarified regulation mechanisms for this isoform, studies have confirmed that modification from multiple transcription factors in the 5' flanking region regulates the amount of PFK-2 expression in developing testis tissue. This isoform has been particularly implicated as being modified and hyper-expressed for prostate cancer cell survival.
Clinical significance
Because this enzyme family maintains rates of glycolysis and gluconeogenesis, it presents great potential for therapeutic action for control of metabolism particularly in diabetes and cancer cells. Data also demonstrates that all of the PFK-2 genes (although the PFKB3 gene response remains the most drastic) were activated by limitations in oxygen.Minchenko, O., Opentanova, I., & Caro, J. (2003). Hypoxic regulation of the 6‐phosphofructo‐2‐kinase/fructose‐2, 6‐bisphosphatase gene family (PFKFB‐1–4) expression in vivo. ''FEBS Letters'', ''554''(3), 264-270. The control of PFK-2/FBP-ase2 activity was found to be linked to heart functioning, particularly forischemia
Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism (to keep tissue alive). Ischemia is generally caused by problems wi ...
, and the control against hypoxia
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the tis ...
. Researchers hypothesize that this responsive characteristic of the PFK-2 genes may be a strong, evolutionary physiological adaptation. However, many human cancer cell types (including leukemia, lung, breast, colon, pancreatic, and ovarian cancers) demonstrate over-expression of PFK3 and/or PFK4; this change in metabolism likely plays a role in the Warburg effect.
Lastly, the ''Pfkfb2'' gene encoding PFK2/FBPase2 protein is linked to the predisposition to schizophrenia.
References
*External links
*6-phosphofructokinase of Arabidopsis thaliana at genome.jp
''This article incorporates text from the public domain
Pfam
Pfam is a database of protein families that includes their annotations and multiple sequence alignments generated using hidden Markov models. The most recent version, Pfam 35.0, was released in November 2021 and contains 19,632 families.
Uses
...
and InterProbr>IPR013079' EC 2.7.1 EC 3.1.3 Glycolysis Genes on human chromosome 10 Enzymes of known structure {{InterPro content, IPR013079