Ferrichrome on:
[Wikipedia]
[Google]
[Amazon]
Ferrichrome is a cyclic hexa-
 The main types of
The main types of
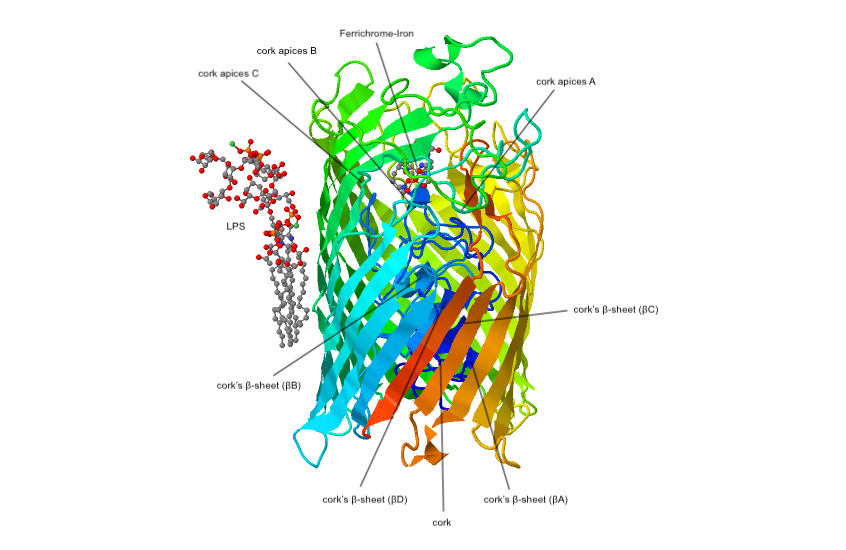 The green ribbons represent β-barrel wall that is 69Å long x 40-45Å diameter that represents the C-terminus residues. It has 22 antiparallel β strands. The blue ribbon in the center is a “cork” which is a distinct domain for the N-terminus residues.
FhuA has L4 strand and its role is to transport ferrichrome into the β-barrel wall. The ferrichrome complex then binds tightly to both the β-barrel wall and the "cork". As a result, these binding triggers two key conformation changes to iron-ferrichrome complex to transfer energy to the cork. This energy transfer results in subsequent conformational changes that transport iron-ferrichrome to the periplasmic pocket which signal a ligand loaded status of the receptor. These subtle shifts disrupt the binding of iron-ferrichrome to the cork which then allows the permeation of the ferrichrome-iron to a putative channel-forming region. The inner wall of the β-barrel provides a series of weak binding sites to pull ferrichrome along. FhuD is a high affinity binding protein in the periplasmic pocket that also aids in unidirectional transport across the cell envelope.
The green ribbons represent β-barrel wall that is 69Å long x 40-45Å diameter that represents the C-terminus residues. It has 22 antiparallel β strands. The blue ribbon in the center is a “cork” which is a distinct domain for the N-terminus residues.
FhuA has L4 strand and its role is to transport ferrichrome into the β-barrel wall. The ferrichrome complex then binds tightly to both the β-barrel wall and the "cork". As a result, these binding triggers two key conformation changes to iron-ferrichrome complex to transfer energy to the cork. This energy transfer results in subsequent conformational changes that transport iron-ferrichrome to the periplasmic pocket which signal a ligand loaded status of the receptor. These subtle shifts disrupt the binding of iron-ferrichrome to the cork which then allows the permeation of the ferrichrome-iron to a putative channel-forming region. The inner wall of the β-barrel provides a series of weak binding sites to pull ferrichrome along. FhuD is a high affinity binding protein in the periplasmic pocket that also aids in unidirectional transport across the cell envelope.
peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
that forms a complex with iron atoms. It is a siderophore
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is no ...
composed of three glycine and three modified ornithine residues with hydroxamate groups N(OH)C(=O)C- The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination.
Ferrichrome was first isolated in 1952, and has been found to be produced by fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
of the genera ''Aspergillus
' () is a genus consisting of several hundred mold species found in various climates worldwide.
''Aspergillus'' was first catalogued in 1729 by the Italian priest and biologist Pier Antonio Micheli. Viewing the fungi under a microscope, Miche ...
'', ''Ustilago
''Ustilago'' is a genus of approximately 200 smut fungi parasitic on grasses.
Uses
''Ustilago maydis'' is eaten as a traditional Mexican food in many parts of the country, and is even available canned. Farmers have even been known to spread th ...
'', and ''Penicillium
''Penicillium'' () is a genus of ascomycetous fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.
Some members of the genus produce pe ...
''. However, at the time there was no understanding regarding its involvement and contribution to iron transport. It was not until 1957 because of Joe Neilands work, where he first noted that Ferrichrome was able to act as an iron transport agent.
Biological function
Ferrichrome is a siderophore, which are metalchelating agent
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
s that have a low molecular mass and are produced by microorganisms and plants growing under low iron conditions. The main function of siderophores is to chelate ferric iron (Fe3+) from insoluble minerals from the environment and make it available for microbial and plant cells. Iron is important in biological functions as it acts as a catalyst in enzymatic processes, as well as for electron transfer, DNA and RNA synthesis, and oxygen metabolism. Although iron is the fourth most abundant element in the earth’s crust, bioavailability of iron in aerobic environments is low due to formation of insoluble ferric hydroxides. Under iron limitation, bacteria scavenge for ferric iron (Fe3+) by up-regulating the secretion of siderophores in order to meet their nutritional requirements. Recent studies have shown that ferrichrome has been used as a tumor- suppressive molecule produced by L. casei
''Lacticaseibacillus casei ''is an organism that belongs to the largest genus in the family ''Lactobacillaceae'', a lactic acid bacteria (LAB), that was previously classified as ''Lactobacillus casei-01''. This bacteria has been identified as facu ...
. The study from the Department of Medicine and Asahikawa Medical University, suggests that ferrichrome has a greater tumor-suppressive effect than other drugs currently used to fight colon cancer, including cisplatin
Cisplatin is a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, br ...
and 5-fluoro-uracil. Ferrichrome also had less of an effect on non-cancerous intestinal cells than the two previously mentioned cancer fighting drugs. It was determined that ferrichrome activated the C-Jun N-terminal kinases
c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family, and are res ...
, which induced apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
. The induction of apoptosis by ferrichrome is reduced by the inhibition of the c-jun N-terminal kinase signaling pathway.
Uptake
Iron is essential for the most important biological processes such as DNA and RNA synthesis, glycolysis, energy generation, nitrogen fixation and photosynthesis, therefore uptake of iron from the environment and transport into the organism are critical life processes for almost all organisms. The problem is when environmental iron is exposed to oxygen it is mineralized to its insoluble ferric oxy hydroxide form which can not be transported into the cells and therefore is not available for use by the cell. To overcome this, bacteria, fungi and some plants synthesize siderophores, and secrete it into an extracellular environment where binding of iron can occur. It is important to note microbes make their own type of siderophore so that they are not competing with other organisms for iron uptake. For example, ''saccharomyces cerevisiae'' is a species of yeast that can uptake the iron bound siderophore through transporters of the ARN family. e3+( siderophore)sup>(n-3)- binds to a receptor-transporter on the cell surface and then is up taken. The exact mechanism how iron enters the cell using these transporters is not understood, but it known that once it enters the cell it accumulates in the cytosol. In ''saccharomyces cerevisiae'', ferrichrome is specifically taken up by ARN1P as it has 2 binding sites and ferrichrome can the higher affinity site through endocytosis. Ferrichrome chelates stay stable in the cell and allow for iron storage, but can be easily mobilized to meet the metabolic needs of the cell. The removal of Fe3+ occurs through the reduction of Fe3+ to Fe2+. The reduction strategy helps in making the iron more aqueous soluble, and allows the iron to become morebioavailable
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
in order for uptake to occur. This is because the Fe2+ product is not able to mineralize like the Fe3+, as it does not bind significantly to the chelate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
that is designed to bind Fe3+. In addition to this, the Fe3+ product can also release Fe2+ from the chelate ligands that was designed to bind Fe3+. Fe2+ has little to no affinity towards the siderophore ligand and this removal is necessary for use and storage. This is because Fe2+ is an intermediate acid, therefore it is not able to bind significantly to the siderophore chelate ligands and can only bind with a much lower affinity. Whereas, Fe3+ is a hard base and can bind to the siderophore chelate ligands with a much higher affinity. The Fe3+ siderophore
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is no ...
complexes are taken up into the bacterial membrane by active transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellul ...
mechanisms. This uptake process is able to recognize different structural features of the siderophores and transport the Fe3+ complexes into the periplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in gram-negative bacteria. Using cryo-electron microscopy it has been found that ...
.
Siderophore Binding
 The main types of
The main types of siderophores
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is no ...
have catecholate, hydroxamate, and carboxylate coordinating ligands. An example of a catecholate siderophore includes enterobactin
Enterobactin (also known as enterochelin) is a high affinity siderophore that acquires iron for microbial systems. It is primarily found in Gram-negative bacteria, such as ''Escherichia coli'' and ''Salmonella typhimurium''.
Enterobactin is the ...
. Examples of hydroxamate siderophores include desferrioxamine
Deferoxamine (DFOA), also known as desferrioxamine and sold under the brand name Desferal, is a medication that binds iron and aluminium. It is specifically used in iron overdose, hemochromatosis either due to multiple blood transfusions or an un ...
, ferrichrome, aerobactin, rhodotorullic acid, and alcaligin. Aerobactin is a carboxylate siderophore as well. The triscatecholate siderophore, enterobactin, has a higher binding affinity of logβ110 = 49 to ferric iron compared to Ferrichrome, which has a binding affinity of logβ110 = 29.07. Therefore, it would outcompete with the other siderophore and bind more of the available environmental Fe3+. It does not bind other metals in high concentration because of its high Fe3+ specificity. The trishydroxamate siderophore, desferrioxamine, has a binding affinity of logβ110 = 30.6 and has a lower binding affinity compared to Ferrichrome. Therefore, the desferrioxamine siderophore can also outcompete Ferrichrome, and bind more of the available environmental Fe3+. However, the bishydroxamate siderophores aerobactin (logβ110 = 22.5), rhodotorullic acid (logβ110 =21.55), and alcaligin (logβ110 = 23.5) will not be able to outcompete with the triscatecholate and trishydroxamate siderophores, since they do not have high Fe3+ specificity. Therefore, they are not able to bind more of the available environmental Fe3+.
Iron in its trivalent state has an electron configuration of d5, therefore, its complexes are preferentially hexacoordinate, quasi octahedral. In terms of the HSAB principle, ferric siderophores have donor atoms that are mainly oxygen and rarely heterocyclic nitrogen. This is because of the ferric ion being a hard Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, and the ferric iron therefore binds more strongly with a hard anionic oxygen donor.
FhuA Uptake Mechanism
E. coli has a receptor protein called FhuA (ferric Hydroxamate). FhuA’s is an energy-coupled transporter and receptor. It is a part of the integral outer membrane proteins and works alongside an energy transducing protein TonB.{{cite journal , vauthors = Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W , title = Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide , journal = Science , volume = 282 , issue = 5397 , pages = 2215–2220 , date = December 1998 , pmid = 9856937 , doi = 10.1126/science.282.5397.2215 , doi-access = free , bibcode = 1998Sci...282.2215F It is involved in the uptake of iron in complex with ferrichrome by binding and transporting ferrichrome-iron across the cell’s outer membrane. The green ribbons represent β-barrel wall that is 69Å long x 40-45Å diameter that represents the C-terminus residues. It has 22 antiparallel β strands. The blue ribbon in the center is a “cork” which is a distinct domain for the N-terminus residues.
FhuA has L4 strand and its role is to transport ferrichrome into the β-barrel wall. The ferrichrome complex then binds tightly to both the β-barrel wall and the "cork". As a result, these binding triggers two key conformation changes to iron-ferrichrome complex to transfer energy to the cork. This energy transfer results in subsequent conformational changes that transport iron-ferrichrome to the periplasmic pocket which signal a ligand loaded status of the receptor. These subtle shifts disrupt the binding of iron-ferrichrome to the cork which then allows the permeation of the ferrichrome-iron to a putative channel-forming region. The inner wall of the β-barrel provides a series of weak binding sites to pull ferrichrome along. FhuD is a high affinity binding protein in the periplasmic pocket that also aids in unidirectional transport across the cell envelope.
The green ribbons represent β-barrel wall that is 69Å long x 40-45Å diameter that represents the C-terminus residues. It has 22 antiparallel β strands. The blue ribbon in the center is a “cork” which is a distinct domain for the N-terminus residues.
FhuA has L4 strand and its role is to transport ferrichrome into the β-barrel wall. The ferrichrome complex then binds tightly to both the β-barrel wall and the "cork". As a result, these binding triggers two key conformation changes to iron-ferrichrome complex to transfer energy to the cork. This energy transfer results in subsequent conformational changes that transport iron-ferrichrome to the periplasmic pocket which signal a ligand loaded status of the receptor. These subtle shifts disrupt the binding of iron-ferrichrome to the cork which then allows the permeation of the ferrichrome-iron to a putative channel-forming region. The inner wall of the β-barrel provides a series of weak binding sites to pull ferrichrome along. FhuD is a high affinity binding protein in the periplasmic pocket that also aids in unidirectional transport across the cell envelope.
See also
Ferrichrome A
Ferrichrome A is a siderophore in the ferrichrome family. Iron is an essential element for the survival and proliferation of organisms. Microorganisms produce and secrete potent iron chelators, also known as siderophores, to aid in the sequestrat ...
References