Dental Porcelain on:
[Wikipedia]
[Google]
[Amazon]
 Dental
Dental
 Dental
Dental porcelain
Porcelain () is a ceramic material made by heating substances, generally including materials such as kaolinite, in a kiln to temperatures between . The strength and translucence of porcelain, relative to other types of pottery, arises main ...
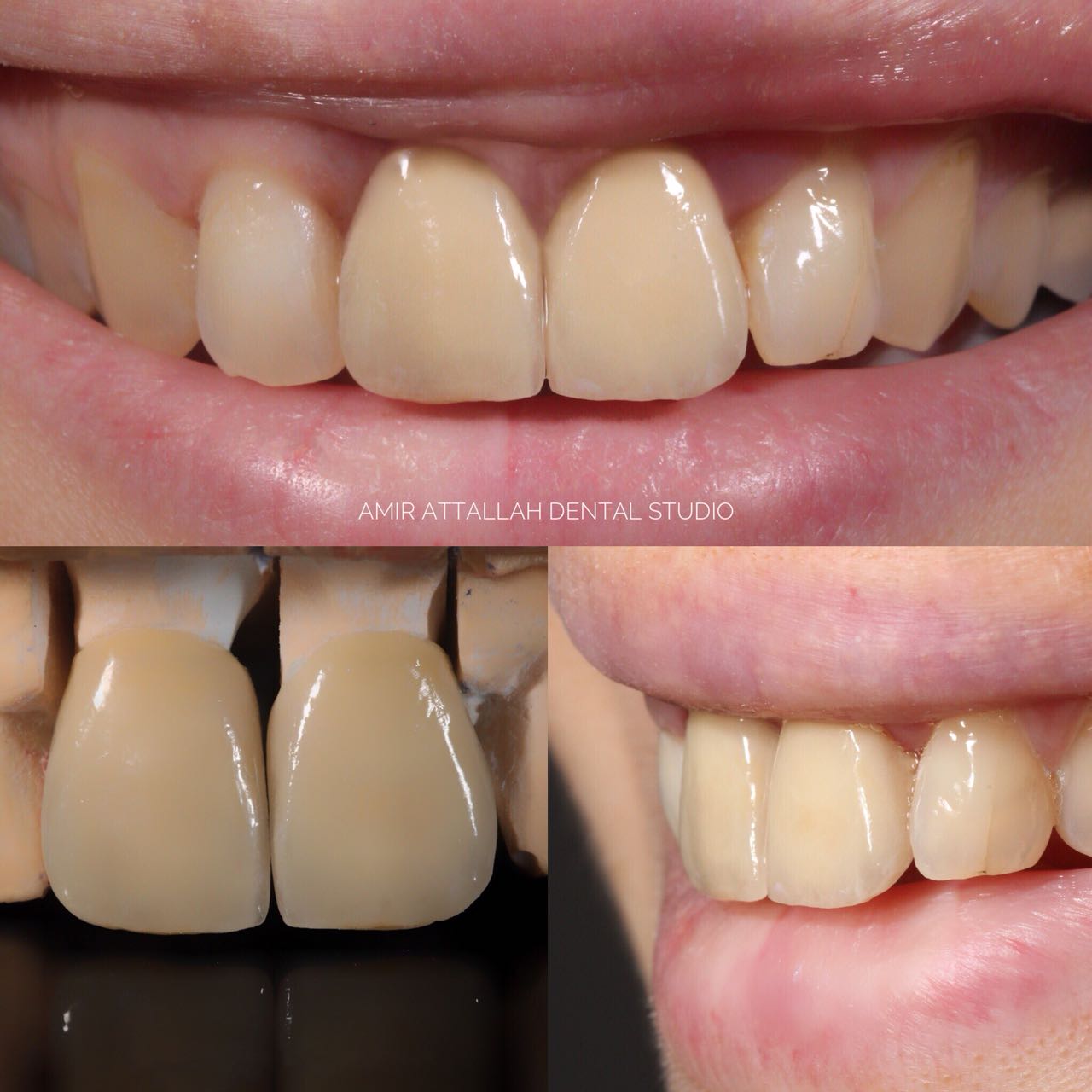
(also known as dental ceramic) is a dental material used by dental technician
A dental technologist (dental laboratory technician) is a member of the dental team who, upon prescription from a dental clinician, constructs custom-made restorative and dental appliances.
There are four major disciplines within dental technol ...
s to create biocompatible
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing de ...
lifelike dental restoration
Dental restoration, dental fillings, or simply fillings are treatments used to restore the function, integrity, and morphology of missing tooth structure resulting from caries or external trauma as well as to the replacement of such structure sup ...
s, such as crowns
A crown is a traditional form of head adornment, or hat, worn by monarchs as a symbol of their power and dignity. A crown is often, by extension, a symbol of the monarch's government or items endorsed by it. The word itself is used, partic ...
, bridges
A bridge is a structure built to span a physical obstacle (such as a body of water, valley, road, or rail) without blocking the way underneath. It is constructed for the purpose of providing passage over the obstacle, which is usually someth ...
, and veneers. Evidence suggests they are an effective material as they are biocompatible
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing de ...
, aesthetic, insoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
and have a hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
of 7 on the Mohs scale. For certain dental prostheses, such as three-unit molars porcelain fused to metal or in complete porcelain group, zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant sta ...
-based restorations are recommended.
The word "ceramic" is derived from the Greek word ''keramos'', meaning "potter's clay". It came from the ancient art of fabricating pottery where mostly clay was fired to form a hard, brittle object; a more modern definition is a material that contains metallic and non-metallic elements (usually oxygen). These materials can be defined by their inherent properties including their hard, stiff, and brittle nature due to the structure of their inter-atomic bonding, which is both ionic and covalent. In contrast, metals are non-brittle (display elastic behavior), and ductile (display plastic behaviour) due to the nature of their inter-atomic metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
. These bonds are defined by a cloud of shared electrons with the ability to move easily when energy is applied. Ceramics can vary in opacity from very translucent to very opaque. In general, the more glassy the microstructure (i.e. noncrystalline) the more translucent it will appear, and the more crystalline, the more opaque.
Composition
Ceramic used in dental application differs in composition from conventional ceramic to achieve optimum aesthetic components such as translucency. As example the composition of dental feldspathic porcelain is as follows: *Kaolin
Kaolinite ( ) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is an important industrial mineral. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral ...
3-5%
* Quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
(silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
) 12-25%
* Feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) felds ...
70-85%
* Metallic colourants 1%
* Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching ...
up to 15%
Classification
Ceramics can be classified based on the following:
Classification by Microstructure
At the microstructural level, ceramics can be defined by the nature of their composition of amorphous-to-crystalline ratio. There can be an infinite variability of the microstructures of materials, but they can be broken down into four basic compositional categories, with a few subgroups: * Composition category 1 – glass-based systems (mainly silica), example is the feldspathic porcelain. * Composition category 2 – glass-based systems (mainly silica) with fillers, usually crystalline (typicallyleucite
Leucite is a rock-forming mineral of the feldspathoid group, silica-undersaturated and composed of potassium and aluminium tectosilicate KAlSi2O6. Crystals have the form of cubic icositetrahedra but, as first observed by Sir David Brewster in ...
or, more recently, lithium disilicate)
* Composition category 3 – crystalline-based systems with glass fillers (mainly alumina)
* Composition category 4 – polycrystalline solids (alumina and zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant sta ...
).
Dental ceramic is generally regarded as biologically inert. However, other toxicities may exist from depleted uranium as well as some of the other accessory materials; in addition, the restoration may increase wear on opposing teeth.
Classification by Processing Technique
* Powder/liquid, glass-based systems * Machinable or pressable blocks of glass-based systems * CAD/CAM or slurry, die-processed, mostly crystalline systemsClassification of crystalline ceramics
Types of Ceramics
The range of dental ceramics determined by their respective firing temperatures are: * Ultra-low Fired below 850 °C - mainly used for shoulder ceramics (aims to combat the problem of shrinkage, specifically at the margins of the prep, when the early sintered ceramic state is fired to produce the final restoration), to correct minor defects and to add colour/shading to restorations * Low fusing Fired between 850 and 950 °C - to prevent the occurrence of distortion, this type of ceramic should not be subjected to multiple firings * Higher fusing This type is used mainly for denture teethLaboratory Procedure
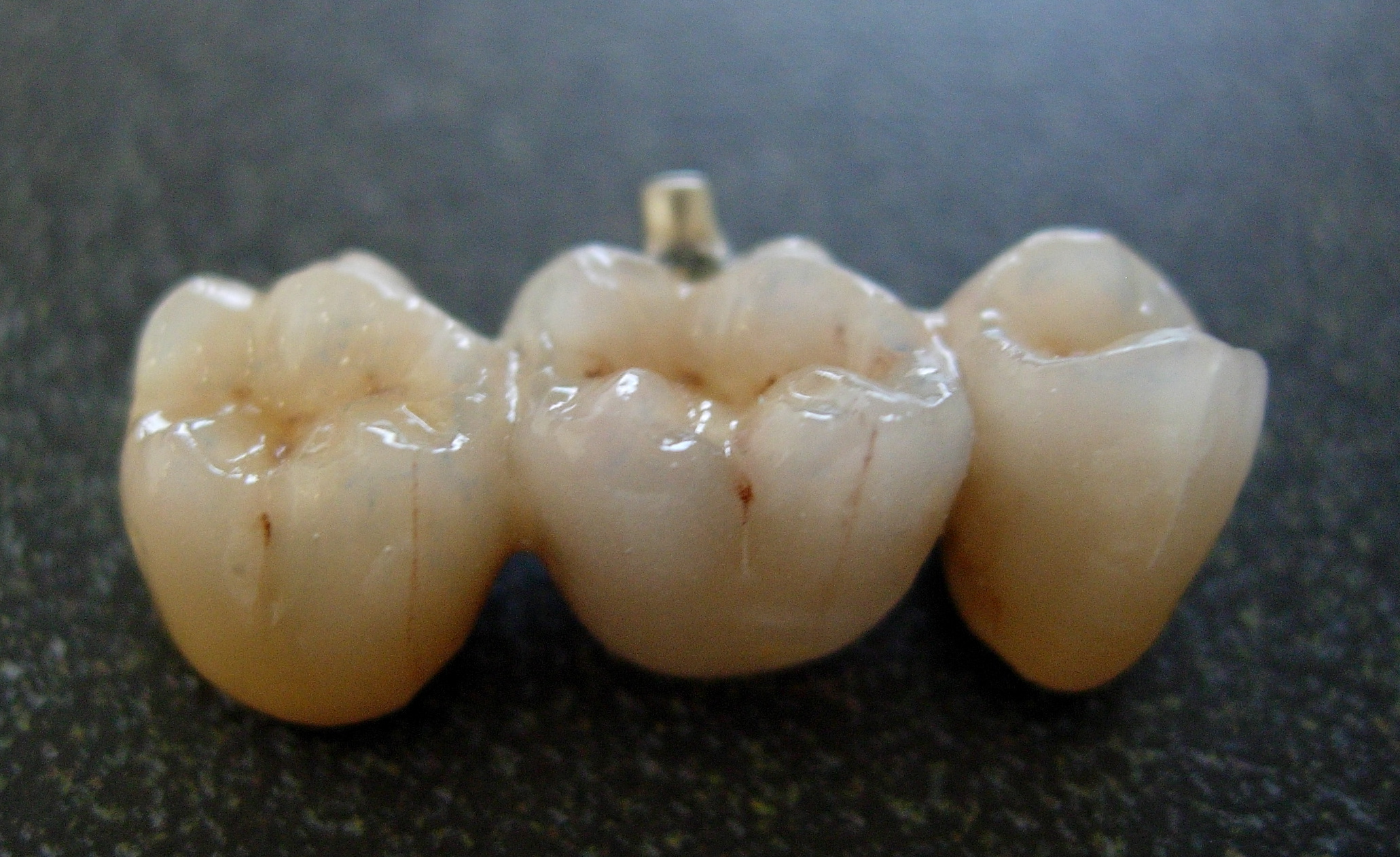
The dentist will usually specify a shade or combination of shades for different parts of the restoration, which in turn corresponds to a set of samples containing the porcelain powder. There are two types of porcelain restorations: * Porcelain fused to metal * Complete porcelain Ceramic restorations can be built on a refractory die, which is a reproduction of a prepared tooth made of a strong material with the ability to withstand high temperatures, or it can be constructed on a metal coping or core. For ceramic fused to metal restorations, the black color of metal is first masked with an opaque layer giving it a shade of white before consecutive layers are built up. The powder corresponding to the desired shade ofdentine
Dentin () (American English) or dentine ( or ) (British English) ( la, substantia eburnea) is a calcified tissue of the body and, along with enamel, cementum, and pulp, is one of the four major components of teeth. It is usually covered by e ...
base is mixed with water before it is fired. Further layers are built up to mimic the natural translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions ...
of the enamel of the tooth
A tooth ( : teeth) is a hard, calcified structure found in the jaws (or mouths) of many vertebrates and used to break down food. Some animals, particularly carnivores and omnivores, also use teeth to help with capturing or wounding prey, t ...
. The porcelain is fused to a semi-precious metal or precious metal, such as gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
, for extra strength.
Systems which use an aluminium oxide, zirconium oxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabi ...
or zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant sta ...
core instead of metal, produces complete porcelain restorations.
Firing
Once the mass has been built up, it is fired to allow fusion of the ceramic particles which in turn forms the completed restoration; the process by which this is done is referred to as ‘baking’. The first bake forces water out and allows the particles to coalesce. During this initial process, a large amount of shrinkage occurs until the mass reaches an almost void-free state; to overcome this the mass is built-up to a size larger than the final restoration will be. The mass is then left to cool slowly to prevent cracking and reduced strength of the final restoration. Adding more layers to build up the restoration to the desired shape and/or size requires the ceramic to undergo further rounds of firing.Staining
Ceramic can also be stained to show tooth morphology such as occlusal fissures and hypoplastic spots. These stains can be incorporated within the ceramic or applied onto the surface.Glazing
Glazing is required to produce a smooth surface and it's the last stage of sealing the surface as it will fill porous areas and prevent wear on opposing teeth. Glazing can be achieved by re-firing the restoration, which fuses outer layers of the ceramic, or by using glazes with lower fusing temperatures; these are applied on the outer surface of the restoration in a thin layer. Any adjustments are then made with polishing rubbers and fine diamonds.Use of CAD-CAM
Recent developments in CAD/CAM dentistry uses special partially sintered ceramic (zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant sta ...
), glass-bonded ceramic or glass-ceramic
Glass-ceramics are polycrystalline materials produced through controlled crystallization of base glass, producing a fine uniform dispersion of crystals throughout the bulk material. Crystallization is accomplished by subjecting suitable glasses to ...
( lithium disilicate) formed into machinable blocks, which are fired again after machining.
By utilising in-office CAD/CAM technology, clinicians are able to design, fabricate and place all-ceramic inlays, onlays, crowns and veneers in a single patient visit. Ceramic restorations produced by this method have demonstrated excellent fit, strength and longevity. Two basic techniques can be used for CAD/CAM restorations:
* Chairside single-visit technique
* Integrated chairside–laboratory CAD/CAM procedure
Ceramic Restorations can be
Ceramic restorations are indicated for most dental applications including: * Veneers * Inlays * Onlays * Crowns * Bridges * Implant supra- and sub-structures * Denture teeth However, each system will have its own set of specific indications and contraindications which can be obtained from the manufacturers guideline.Contraindications for Ceramic Restorations
Ceramic restorations are contraindicated when a patient presents with the following: * Parafunction; individuals who suffer from bruxism or clenching * Short clinical crown * Immature teeth * Unfavourableocclusion
Occlusion may refer to:
Health and fitness
* Occlusion (dentistry), the manner in which the upper and lower teeth come together when the mouth is closed
* Occlusion miliaria, a skin condition
* Occlusive dressing, an air- and water-tight trauma ...
* Supragingival preparations (when used alongside adhesive cements)
Other uses
Denture Teeth
Poly(methyl methacrylate) (PMMA) is the material of choice for denture teeth, however ceramic denture teeth have been, and still are used for this purpose. The main benefit associated with the use of ceramic teeth is their superior wear resistance. There are however a number of disadvantages to using ceramic for denture teeth including their inability to form chemical bonds with the PMMA denture base; rather, ceramic teeth are attached to the base via mechanical retention which increases the chance of debonding during use over time. Additionally, they are more likely to fracture due to their brittle nature.Endodontic Posts
Ceramic can be used in the construction of non-metallic posts, however, it is a brittle material and as such may fracture within theroot canal
A root canal is the naturally occurring anatomic space within the root of a tooth. It consists of the pulp chamber (within the coronal part of the tooth), the main canal(s), and more intricate anatomical branches that may connect the root ...
or may cause fracture of the root due to its increased strength. Another disadvantage is that once placed, removal may not be possible.
References
{{reflist Dental materials Porcelain