Dendrite (crystal) on:
[Wikipedia]
[Google]
[Amazon]
 A crystal dendrite is a
A crystal dendrite is a

 A crystal dendrite is a
A crystal dendrite is a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
that develops with a typical multi-branching form. The name comes from the Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
word dendron (δενδρον) which means "tree", since the crystal's structure resembles that of a tree. These crystals can be synthesised by using a supercooled
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
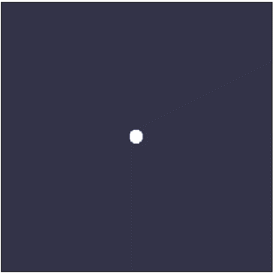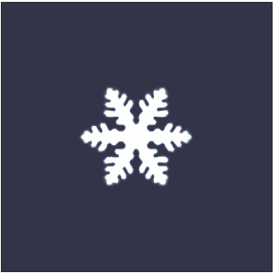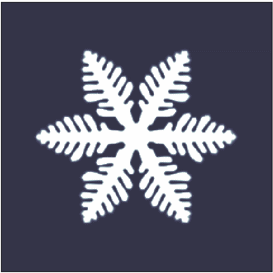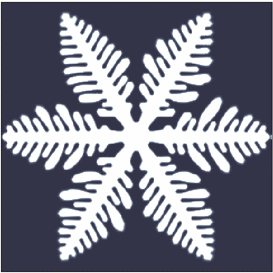
pure liquid, however they are also quite common in nature. The most common crystals in nature exhibit dendritic growth are snowflake
A snowflake is a single ice crystal that has achieved a sufficient size, and may have amalgamated with others, which falls through the Earth's atmosphere as snow.Knight, C.; Knight, N. (1973). Snow crystals. Scientific American, vol. 228, no. ...
s and frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor in an above- freezing atmosphere coming in contact with a solid surface whose temperature is below freezing, and resulting in a phase change from water vapor (a g ...
on windows, but many mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ...
s and metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s can also be found in dendritic structures.
History


Maximum velocity principle
The first dendritic patterns were discovered inpalaeontology
Paleontology (), also spelled palaeontology or palæontology, is the scientific study of life that existed prior to, and sometimes including, the start of the Holocene epoch (roughly 11,700 years before present). It includes the study of fossi ...
and are often mistaken for fossil
A fossil (from Classical Latin , ) is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved ...
s because of their appearance. The first theory for the creation of these patterns was published by Nash and Glicksman in 1974, they used a very mathematical method and derived a non-linear integro-differential equation for a classical needle growth. However they only found an inaccurate numerical solution close to the tip of the needle and they found that under a given growth condition, the tip velocity has a unique maximum value. This became known as the maximum velocity principle (MVP) but was ruled out by Glicksman and Nash themselves very quickly. In the following two years Glicksman improved the numerical methods used, but did not realise the non-linear integro-differential equation had no mathematical solutions making his results meaningless.
Marginal stability hypothesis
Four years later, in 1978,Langer
Langer is a surname originally of German origin. For the etymology, meaning, and pronunciation of the name, and for the Hiberno-English slang word, see Wiktionary.
People with the family name Langer include:
Academics and scientists
* Alois Lang ...
and Müller-Krumbhaar proposed the marginal stability hypothesis (MSH). This hypothesis used a stability parameter σ which depended on the thermal diffusivity
In heat transfer analysis, thermal diffusivity is the thermal conductivity divided by density and specific heat capacity at constant pressure. It measures the rate of transfer of heat of a material from the hot end to the cold end. It has the SI ...
, the surface tension and the radius of the tip of the dendrite. They claimed a system would be unstable for small σ causing it to form dendrites. At the time however Langer and Müller-Krumbhaar were unable to obtain a stability criterion for certain growth systems which lead to the MSH theory being abandoned.
Microscopic solvability condition
A decade later several groups of researchers went back to the Nash-Glicksman problem and focused on simplified versions of it. Through this they found that the problem for isotropic surface tension had no solutions. This result meant that a system with a steady needle growth solution necessarily needed to have some type of anisotropic surface tension. This breakthrough lead to the microscopic solvability condition theory (MSC), however this theory still failed since although for isotropic surface tension there could not be a steady solution, it was experimentally shown that there were nearly steady solutions which the theory did not predict.Macroscopic continuum model
Nowadays the best understanding for dendritic crystals comes in the form of the macroscopic continuum model which assumes that both the solid and the liquid parts of the system are continuous media and the interface is a surface. This model uses the microscopic structure of the material and uses the general understanding of nucleation to accurately predict how a dendrite will grow.Dendrite formation
Dendrite formation starts with some nucleation, i.e. the first appearance of solid growth, in thesupercooled
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
liquid. This formation will at first grow spherically until this shape is no longer stable. This instability has two causes: anisotropy
Anisotropy () is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's phys ...
in the surface energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energ ...
of the liquid-solid interface and the attachment kinetics of particles to crystallographic planes when they have formed.
On the solid-liquid interface, we can define a surface energy which is the excess energy at the liquid-solid interface to accommodate the structural changes at the interface.
For a spherical interface, the Gibbs-Thomson equation then gives a melting point depression compared to a flat interface , which has the relation
where is the radius of the sphere. This curvature undercooling, the effective lowering of the melting point at the interface, sustains the spherical shape for small radii.
However, anisotropy in the surface energy implies that the interface will deform to find the energetically most favourable shape. For cubic symmetry in 2D we can express this anisotropy int the surface energy as
This gives rise to a surface stiffness