demethylation on:
[Wikipedia]
[Google]
[Amazon]
Demethylation is the chemical process resulting in the removal of a
 Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to
Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to 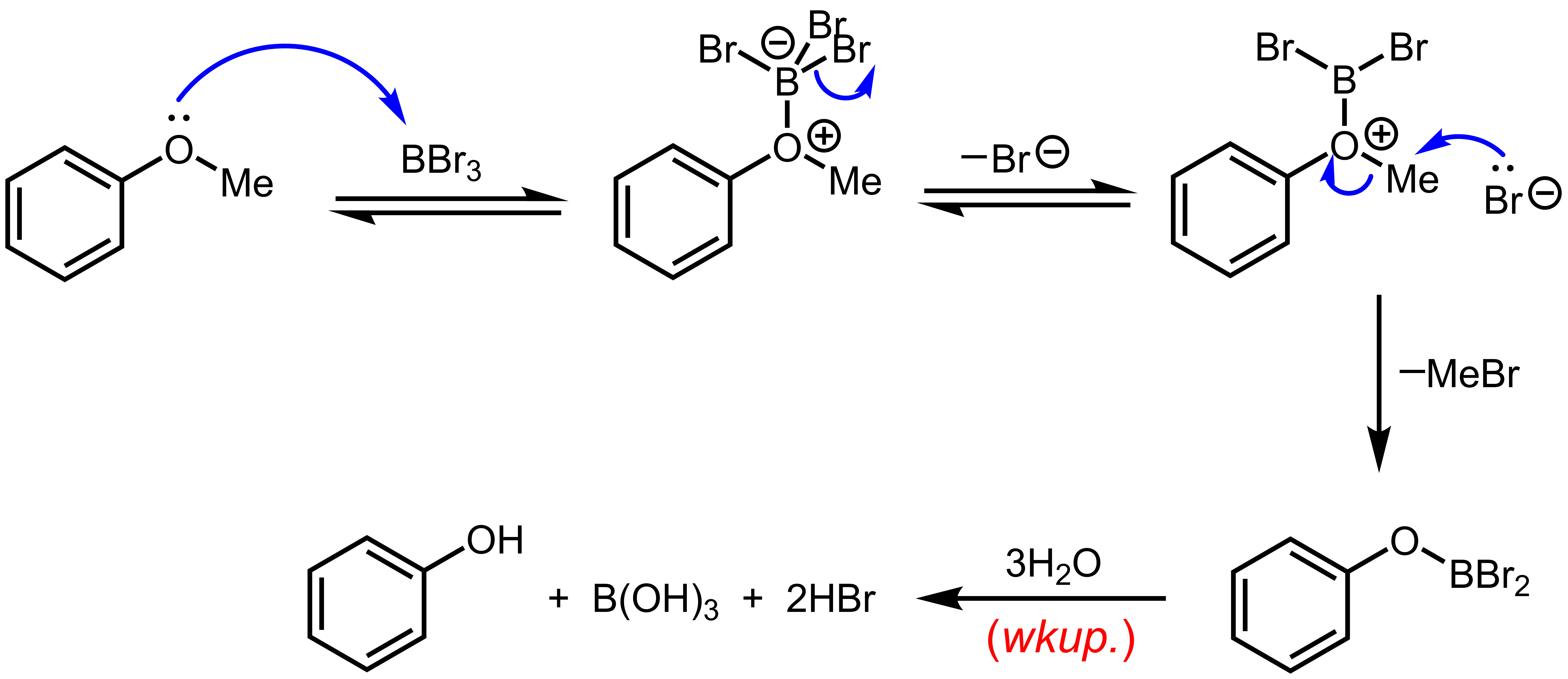 Methyl esters also are susceptible to demethylation, which is usually achieved by
Methyl esters also are susceptible to demethylation, which is usually achieved by  A mixture of
A mixture of
methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
(CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms.
The counterpart of demethylation is methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
.
In biochemistry
Inbiochemical
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology ...
systems, the process of demethylation is catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
by demethylase
Demethylases are enzymes that remove methyl (CH3) groups from nucleic acids, proteins (particularly histones), and other molecules. Demethylases are important epigenetic proteins, as they are responsible for transcriptional regulation of the geno ...
s. These enzymes oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
N-methyl groups, which occur in histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn ar ...
s and some forms of DNA:
:R2N-CH3 + O → R2N-H + CH2O
One such oxidative enzyme family is the cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compo ...
. Alpha-ketoglutarate-dependent hydroxylase Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Fun ...
s are active for demethylation of DNA, operating by a similar pathway. These reactions exploit the weak C-H bond adjacent to amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
.
In particular, 5-methylcytosines in DNA can be demethylated by TET enzymes
The TET enzymes are a family of ten-eleven translocation (TET) methylcytosine dioxygenases. They are instrumental in DNA demethylation. 5-Methylcytosine (see first Figure) is a methylated form of the DNA base cytosine (C) that often regulates gen ...
as illustrated in the figure. TET enzymes are dioxygenase
Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine tri ...
s in the family of alpha-ketoglutarate-dependent hydroxylases Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Fun ...
. A TET enzyme is an alpha-ketoglutarate (α-KG) dependent dioxygenase that catalyses an oxidation reaction by incorporating a single oxygen atom from molecular oxygen (O2) into its substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
, 5-methylcytosine in DNA (5mC), to produce the product 5-hydroxymethylcytosine in DNA. This conversion is coupled with the oxidation of the co-substrate
A cofactor is a non- protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" tha ...
α-KG to succinate and carbon dioxide (see figure).
The first step involves the binding of α-KG and 5-methylcytosine to the TET enzyme active site. The TET enzymes each harbor a core catalytic domain with a double-stranded β-helix fold that contains the crucial metal-binding residues found in the family of Fe(II)/α-KG- dependent oxygenases
An oxygenase is any enzyme that redox, oxidizes a Substrate (biochemistry), substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their Enzyme Commission number, EC number i ...
. α-KG coordinates as a bidentate ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
(connected at two points) to Fe(II)
In chemistry, iron(II) refers to the element iron in its +2 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe2+.
The adjective ferrous or the prefix ferro- is often used to s ...
(see figure), while the 5mC is held by a noncovalent force in close proximity. The TET active site contains a highly conserved triad motif, in which the catalytically-essential Fe(II) is held by two histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the ...
residues and one aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α- amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pr ...
residue (see figure). The triad binds to one face of the Fe center, leaving three labile sites available for binding α-KG and O2 (see figure). TET then acts to convert 5-methylcytosine to 5-hydroxymethylcytosine, while α-ketoglutarate is converted to succinate and CO2.
Demethylation of some sterol
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the g ...
s are steps in the biosynthesis of testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteris ...
and cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membr ...
. Methyl groups are lost as formate
Formate ( IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ...
.
During embryogenesis
An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm ...
in the mouse, about 20 million 5-methylcytosines are demethylated in a six-hour period just after fertilization of an egg by a sperm to form a zygote.
In organic chemistry
Demethylation often refers to cleavage ofethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
, especially aryl ethers, although there are some exceptions, such as ''N''-demethylation of amines (e.g. imipramine
Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) mainly used in the treatment of depression. It is also effective in treating anxiety and panic disorder. The drug is also used to treat bedwetting. ...
to desipramine
Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activiti ...
).
Cleaving methyl ethers
Aryl methyl ethers are pervasive inlignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity ...
and many derived compounds. The demethylation of these materials has been the subject of much effort. The reaction typically requires harsh conditions or harsh reagents. For example, the methyl ether in vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now u ...
can be removed by heating near with strong base. Stronger nucleophiles
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
such as diorganophosphides (LiPPh2) also cleave aryl ethers under milder conditions. Other strong nucleophiles that have been employed include thiolate salts like EtSNa.
Acidic conditions can also be used. Historically, aryl methyl ethers, including natural products such as codeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically use ...
(''O''-methylmorphine), have been demethylated by heating the substance in molten pyridine hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative ...
(melting point ) at , sometimes with excess hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
, in a process known as the ''Zeisel–Prey ether cleavage''. Quantitative analysis for aromatic methyl ethers can be performed by argentometric determination of the ''N''-methylpyridinium chloride formed. The mechanism of this reaction starts with proton transfer from pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is oft ...
ion to the aryl methyl ether, a highly unfavorable step (''K'' < 10–11) that accounts for the harsh conditions required, given the much weaker acidity of pyridinium ( p''K''a = 5.2) compared to the protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
aryl methyl ether (an arylmethyloxonium ion, p''K''a = –6.7 for aryl = Ph). This is followed by SN2 attack of the arylmethyloxonium ion at the methyl group by either pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
or chloride ion (depending on the substrate) to give the free phenol and, ultimately, ''N''-methylpyridinium chloride, either directly or by subsequent methyl transfer from methyl chloride to pyridine. Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to
Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reaction ...
in a solution of hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room tempe ...
or hydrogen iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under s ...
in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
(boiling point ) or concentrated hydrobromic or hydroiodic acid
Hydroiodic acid (or hydriodic acid) is an aqueous solution of hydrogen iodide (HI). It is a strong acid, one that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI.
Reactions
Hyd ...
. The cleavage of ethers by hydrobromic or hydroiodic acid proceeds by a very similar mechanism, in which the highly acidic HBr or HI serves to protonate the ether, followed by displacement by bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardan ...
or iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine def ...
, both of which are excellent nucleophiles. A slightly milder set of conditions uses cyclohexyl iodide (CyI, 10.0 equiv) in ''N'',''N''-dimethylformamide to generate a small amount of hydrogen iodide ''in situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...
''. Boron tribromide
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are amber to red/brown, due to weak bromine contamination. It is decomposed by water and alcohols.
Chemical properties
Boron ...
, which can be used at room temperature or below, is a more specialized reagent for the demethylation of aryl methyl ethers. The mechanism of ether dealkylation proceeds via the initial reversible formation of a Lewis acid-base adduct between the strongly Lewis acidic BBr3 and the Lewis basic ether. This Lewis adduct can reversibly dissociate to give a dibromoboryl oxonium cation and Br–. Rupture of the ether linkage occurs through the subsequent nucleophilic attack on the oxonium species by Br– to yield an aryloxydibromoborane and methyl bromide. Upon completion of the reaction, the phenol is liberated along with boric acid (H3BO3) and hydrobromic acid (aq. HBr) upon hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
of the dibromoborane derivative during aqueous workup.
 Methyl esters also are susceptible to demethylation, which is usually achieved by
Methyl esters also are susceptible to demethylation, which is usually achieved by saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains ...
. Highly specialized demethylations are abundant, such as the Krapcho decarboxylation
The Krapcho decarboxylation is the chemical reaction of esters with halide anions. The ester must contain an electron-withdrawing group in the beta position, such as β-ketoesters, malonic esters, α-cyanoesters, or α-sulfonylesters. It works be ...
:
:anethole
Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It is in the class of ph ...
, KOH, and alcohol was heated in an autoclave. Although the product of this reaction was the expected anol
Anol, also known as ''para''-hydroxypropenylbenzene, is a simple phenol that was derived via demethylation from anethole, an estrogenic constituent of anise and fennel, by Sir Charles Dodds in 1937. It was reported to possess extremely potent es ...
, a highly reactive dimerization product in the mother liquors called dianol was also discovered by Charles Dodds
Sir Edward Charles Dodds, 1st Baronet (13 October 1899 – 16 December 1973) was a British biochemist.
Personal life
He was born in Liverpool in 1899, the only child of Ralph Edward Dodds, a shoe retailer, and Jane (née Pack) Dodds. The family ...
.
''N''-demethylation
''N''-demethylation of 3° amines is by thevon Braun reaction
The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide.
An example is the reaction of ''N'',''N''-dimethyl-1-naphthylamine:
These days, most chemist have replaced cyanogen b ...
, which uses BrCN as the reagent to give the corresponding ''nor- In chemical nomenclature, nor- is a prefix to name a structural analog that can be derived from a parent compound by the removal of one carbon atom along with the accompanying hydrogen atoms. The nor-compound can be derived by removal of a , , or ...
'' derivatives. A modern variation of the von Braun reaction was developed, where BrCN was superseded by ethyl chloroformate
Ethyl chloroformate is the ethyl ester of chloroformic acid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides.
Preparation
Ethyl chlorofo ...
. The preparation of Paxil
Paroxetine, sold under the brand names Paxil and Seroxat among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder ...
from arecoline
Arecoline () is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm ('' Areca catechu''). It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy ...
is an application of this reaction, as well as the synthesis of GSK-372,475, for example.
See also
*Methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
, the addition of a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
to a substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
References
{{Reflist, 30em Gene expression Organic reactions