Deep Carbon Cycle on:
[Wikipedia]
[Google]
[Amazon]
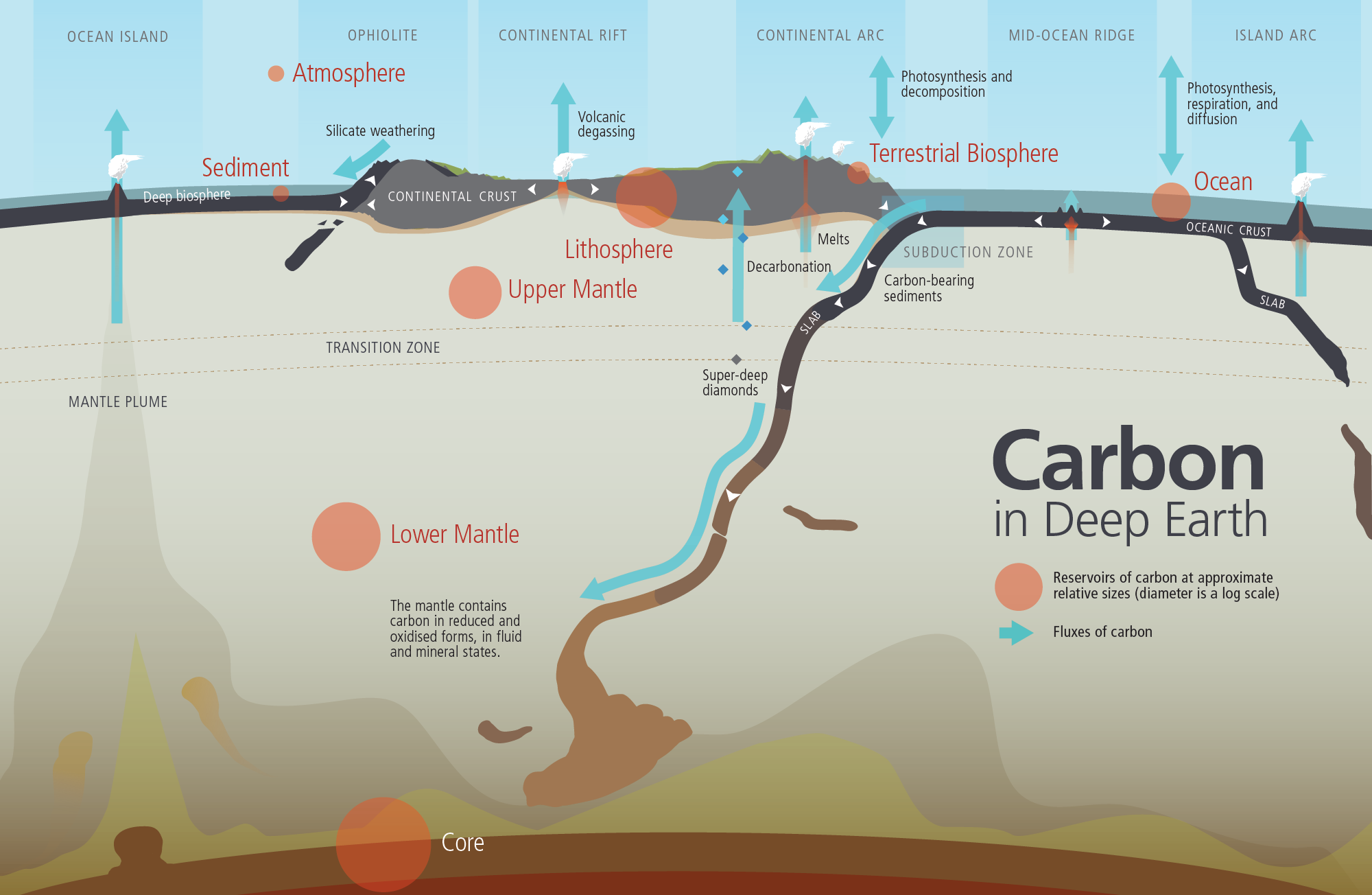 The deep carbon cycle is
The deep carbon cycle is
 Nonetheless, polymorphism alters carbonate compounds' stability at different depths within the Earth. To illustrate, laboratory simulations and
Nonetheless, polymorphism alters carbonate compounds' stability at different depths within the Earth. To illustrate, laboratory simulations and
File:Flux of crustal material in the mantle.jpg, Movement of oceanic plates—which carry carbon compounds—through the mantle
File:Two models.jpg,
File:Speeds of seismic waves.svg, Analysis of shear wave velocities has played an integral role in the development of knowledge about carbon's existence in the core
File:Carbon tetrahedral oxygen.png, Diagram of carbon tetrahedrally bonded to oxygen
 The deep carbon cycle is
The deep carbon cycle is geochemical cycle In Earth science, a geochemical cycle is the pathway that chemical elements take in the surface and crust of the Earth. The term "geochemical" tells us that geological and chemical factors are all included. The migration of heated and compressed che ...
(movement) of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
through the Earth's mantle and core
Core or cores may refer to:
Science and technology
* Core (anatomy), everything except the appendages
* Core (manufacturing), used in casting and molding
* Core (optical fiber), the signal-carrying portion of an optical fiber
* Core, the centra ...
.
It forms part of the carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major componen ...
and is intimately connected to the movement of carbon in the Earth's surface and atmosphere. By returning carbon to the deep Earth, it plays a critical role in maintaining the terrestrial conditions necessary for life to exist. Without it, carbon would accumulate in the atmosphere, reaching extremely high concentrations over long periods of time.
Because the deep Earth is inaccessible to drilling, not much is conclusively known about the role of carbon in it. Nonetheless, several pieces of evidence—many of which come from laboratory simulations of deep Earth conditions—have indicated mechanisms for the element's movement down into the lower mantle, as well as the forms that carbon takes at the extreme temperatures and pressures of this layer. Furthermore, techniques like seismology
Seismology (; from Ancient Greek σεισμός (''seismós'') meaning "earthquake" and -λογία (''-logía'') meaning "study of") is the scientific study of earthquakes and the propagation of elastic waves through the Earth or through other ...
have led to greater understanding of the potential presence of carbon in the Earth's core. Studies of the composition of basaltic magma
Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natural sa ...
and the flux of carbon dioxide out of volcanoes reveals that the amount of carbon in the mantle is greater than that on the Earth's surface by a factor of one thousand.
Quantity of carbon
There are about 44,000 gigatonnes of carbon in the atmosphere and oceans. A gigatonne is one billionmetric tonnes
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton (United States c ...
, equivalent to the mass of water in over 400,000 Olympic-size swimming pools. Large as this quantity is, it only amounts to a small fraction of one percent of Earth's carbon. Over 90% may reside in the core, most of the rest being in the crust and mantle.
In the photosphere of the Sun, carbon is the fourth most abundant element. The Earth likely started with a similar ratio but lost a lot of it to evaporation as it accreted. Even accounting for evaporation, however, the silicates making up the crust and mantle of the Earth have a carbon concentration that is five to ten times less than in CI chondrites, a form of meteor that is believed to represent the composition of the solar nebula before the planets formed. Some of this carbon may have ended up in the core. Depending on the model, carbon is predicted to contribute between 0.2 and 1 percent by weight in the core. Even at the lower concentration, this would account for half Earth's carbon.
Estimates of the carbon content in the upper mantle
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust (at about under the oceans and about under the continents) and ends at the top of the lower mantle at . Temperatures range from appr ...
come from measurements of the chemistry of mid-ocean ridge
A mid-ocean ridge (MOR) is a seafloor mountain system formed by plate tectonics. It typically has a depth of about and rises about above the deepest portion of an ocean basin. This feature is where seafloor spreading takes place along a diver ...
basalts
Basalt (; ) is an aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the surface of a rocky planet or moon. More than 90% of ...
(MORBs). These must be corrected for degassing of carbon and other elements. Since the Earth formed, the upper mantle has lost 40–90% of its carbon by evaporation and transport to the core in iron compounds. The most rigorous estimate gives a carbon content of 30 parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, th ...
(ppm). The lower mantle is expected to be much less depleted – about 350 ppm.
Lower mantle
Carbon principally enters the mantle in the form ofcarbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
-rich sediments on tectonic plates
Plate tectonics (from the la, label=Late Latin, tectonicus, from the grc, τεκτονικός, lit=pertaining to building) is the generally accepted scientific theory that considers the Earth's lithosphere to comprise a number of large ...
of ocean crust, which pull the carbon into the mantle upon undergoing subduction. Not much is known about carbon circulation in the mantle, especially in the deep Earth, but many studies have attempted to augment our understanding of the element's movement and forms within said region. For instance, a 2011 study demonstrated that carbon cycling extends all the way to the lower mantle. The study analysed rare, super-deep diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, ...
s at a site in Juina, Brazil, determining that the bulk composition of some of the diamonds' inclusions matched the expected result of basalt melting and crystallisation
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
under lower mantle temperatures and pressures. Thus, the investigation's findings indicate that pieces of basaltic oceanic lithosphere act as the principal transport mechanism for carbon to Earth's deep interior. These subducted carbonates can interact with lower mantle silicates and metals, eventually forming super-deep diamonds like the one found.
Carbonates descending to the lower mantle form other compounds besides diamonds. In 2011, carbonates were subjected to an environment similar to that of 1800 km deep into the Earth, well within the lower mantle. Doing so resulted in the formations of magnesite, siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). It takes its name from the Greek word σίδηρος ''sideros,'' "iron". It is a valuable iron mineral, since it is 48% iron and contains no sulfur or phosphorus. Zinc, magnesium and ...
, and numerous varieties of graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on lar ...
. Other experiments—as well as petrologic
Petrology () is the branch of geology that studies rocks and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together ...
observations—support this claim, finding that magnesite is actually the most stable carbonate phase in the majority of the mantle. This is largely a result of its higher melting temperature. Consequently, scientists have concluded that carbonates undergo reduction as they descend into the mantle before being stabilised at depth by low oxygen fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas whic ...
environments. Magnesium, iron, and other metallic compounds act as buffers throughout the process. The presence of reduced, elemental forms of carbon like graphite would indicate that carbon compounds are reduced as they descend into the mantle.
 Nonetheless, polymorphism alters carbonate compounds' stability at different depths within the Earth. To illustrate, laboratory simulations and
Nonetheless, polymorphism alters carbonate compounds' stability at different depths within the Earth. To illustrate, laboratory simulations and density functional theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
calculations suggest that tetrahedrally-coordinated carbonates are most stable at depths approaching the core–mantle boundary
The core–mantle boundary (CMB) of Earth lies between the planet's silicate mantle and its liquid iron-nickel outer core. This boundary is located at approximately 2,891 km (1,796 miles) depth beneath Earth's surface. The boundary is observed ...
. A 2015 study indicates that the lower mantle's high pressures cause carbon bonds to transition from sp2 to sp3 hybridised orbitals, resulting in carbon tetrahedrally bonding to oxygen. CO3 trigonal groups cannot form polymerisable networks, while tetrahedral CO4 can, signifying an increase in carbon's coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central io ...
, and therefore drastic changes in carbonate compounds' properties in the lower mantle. As an example, preliminary theoretical studies suggest that high pressures cause carbonate melt viscosity to increase; the melts' lower mobility as a result of the property changes described is evidence for large deposits of carbon deep into the mantle.
Accordingly, carbon can remain in the lower mantle for long periods of time, but large concentrations of carbon frequently find their way back to the lithosphere. This process, called carbon outgassing, is the result of carbonated mantle undergoing decompression melting, as well as mantle plume
A mantle plume is a proposed mechanism of convection within the Earth's mantle, hypothesized to explain anomalous volcanism. Because the plume head partially melts on reaching shallow depths, a plume is often invoked as the cause of volcanic hot ...
s carrying carbon compounds up towards the crust. Carbon is oxidised upon its ascent towards volcanic hotspots, where it is then released as CO2. This occurs so that the carbon atom matches the oxidation state of the basalts erupting in such areas.
Core
Although the presence of carbon in the Earth's core is well-constrained, recent studies suggest large inventories of carbon could be stored in this region. Shear (S) waves moving through the inner core travel at about fifty percent of the velocity expected for most iron-rich alloys. Considering the core's composition is widely believed to be an alloy of crystalline iron with a small amount of nickel, this seismographic anomaly points to another substance's existence within the region. One theory postulates that such a phenomenon is the result of various light elements, including carbon, in the core. In fact, studies have utiliseddiamond anvil cell
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It enables the compression of a small (sub-millimeter-sized) piece of material to extreme pressures, typically up to around 1 ...
s to replicate the conditions in the Earth's core, the results of which indicate that iron carbide
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front ...
(Fe7C3) matches the inner core's sound and density velocities considering its temperature and pressure profile. Hence, the iron carbide model could serve as evidence that the core holds as much as 67% of the Earth's carbon. Furthermore, another study found that carbon dissolved in iron and formed a stable phase with the same Fe7C3 composition—albeit with a different structure than the one previously mentioned. Hence, although the amount of carbon potentially stored in the Earth's core is not known, recent research indicates that the presence of iron carbides could be consistent with geophysical observations.
Fluxes
See also
*Deep Carbon Observatory
The Deep Carbon Observatory (DCO) is a global research program designed to transform understanding of carbon's role in Earth. DCO is a community of scientists, including biologists, physicists, geoscientists and chemists, whose work crosses sever ...
* Geochemistry of carbon
References
Further reading
* * * {{biogeochemical cycle Carbon cycle Geochemistry Earth Plate tectonics