Decarbonylation on:
[Wikipedia]
[Google]
[Amazon]
Decarbonylation is a type of organic reaction that involves loss of CO. It is often an undesirable reaction since it represents a degradation. In the chemistry of
 Silacarboxylic acids (R3SiCOOH) undergo decarbonylation upon heating or treatment with base and have been investigated as carbon monoxide generating molecules.
Silacarboxylic acids (R3SiCOOH) undergo decarbonylation upon heating or treatment with base and have been investigated as carbon monoxide generating molecules.
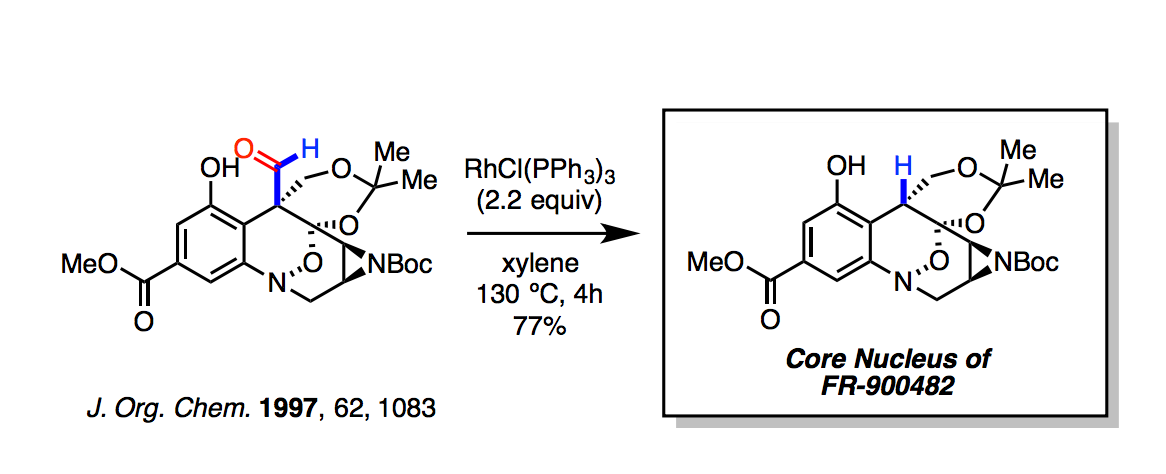 Decarbonylations are of interest in the conversions of sugars.
Decarbonylations are of interest in the conversions of sugars.
metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe ...
s, decarbonylation describes a substitution process, whereby a CO ligand is replaced by another ligand.
Organic chemistry
In the absence of metal catalysts, decarbonylation (vs decarboxylation) is rarely observed in organic chemistry. One exception is the decarbonylation offormic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
:
:HCO2H → CO + H2O
The reaction is induced by sulfuric acid, which functions as both a catalyst and a dehydrating agent. Via this reaction, formic acid is occasionally employed as a source of CO in the laboratory in lieu of cylinders of this toxic gas. With strong heating, formic acid and some of its derivatives may undergo decarbonylation, even without adding a catalyst. For instance, dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the maj ...
slowly decomposes to give dimethylamine and carbon monoxide when heated to its boiling point (154 °C). Some derivatives of formic acid, like formyl chloride, undergo spontaneous decarbonylation at room temperature (or below).
Reactions involving oxalyl chloride
Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.
Preparation
Oxalyl chloride was first prepared in 189 ...
(COCl)2 (e.g., hydrolysis, reaction with carboxylic acids, Swern oxidation, etc.) often liberate both carbon dioxide and carbon monoxide via a fragmentation process.
α-Hydroxy acids, e.g. (lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natur ...
and glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula HOCH2CO2H) is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate ( ...
) undergo decarbonylation when treated with catalytic concentrated sulfuric acid, by the following mechanism:
 Silacarboxylic acids (R3SiCOOH) undergo decarbonylation upon heating or treatment with base and have been investigated as carbon monoxide generating molecules.
Silacarboxylic acids (R3SiCOOH) undergo decarbonylation upon heating or treatment with base and have been investigated as carbon monoxide generating molecules.
Aldehyde decarbonylation
A common transformation involves the conversion ofaldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s to alkane
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms tha ...
s.Kreis, M.; Palmelund, A.; Bunch, L.; Madsen, R., "A General and Convenient Method for the Rhodium-Catalyzed Decarbonylation of Aldehydes", Advanced Synthesis & Catalysis 2006, 348, 2148-2154.
:RCHO → RH + CO
Decarbonylation can be catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
by soluble metal complexes. These reactions proceed via the intermediacy of metal acyl hydride
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl g ...
s. An example of this is the Tsuji–Wilkinson decarbonylation reaction
The Tsuji–Wilkinson decarbonylation reaction is a method for the decarbonylation of aldehydes and some acyl chlorides. The reaction name recognizes , whose team first reported the use of Wilkinson's catalyst (RhCl(PPh3)3) for these reactions: ...
using Wilkinson's catalyst. (Strictly speaking, the noncatalytic version of this reaction results in the formation of a rhodium carbonyl complex rather than free carbon monoxide.) This reaction is generally carried out on small scale in the course of a complex natural product total synthesis, because although this reaction is very efficient at slightly elevated temperatures (e.g., 80 °C) when stoichiometric rhodium is used, catalyst turnover via extrusion of CO requires dissociation of a very stable rhodium carbonyl complex and temperatures exceeding 200 °C are required. This conversion is of value in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, where decarbonylation is an otherwise rare reaction.
 Decarbonylations are of interest in the conversions of sugars.
Decarbonylations are of interest in the conversions of sugars.
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s and other carbonyl-containing functional groups are more resistant to decarbonylation than are aldehydes.
:
Pericyclic reactions
Somecyclic molecule
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where al ...
s containing a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
undergo a cheletropic extrusion reaction, leaving new carbon–carbon π bonds on the remaining structure. This reaction can be spontaneous, as in the synthesis of hexaphenylbenzene
Hexaphenylbenzene is an aromatic compound composed of a benzene ring substituted with six phenyl rings. It is a colorless solid. The compound is the parent member of a wider class of hexaarylbenzenes, which are mainly of theoretical interest.
Pr ...
. Cyclopropenone
Cyclopropenone is an organic compound with molecular formula C3H2O consisting of a cyclopropene carbon framework with a ketone functional group. It is a colorless, volatile liquid that boils near room temperature. Neat cyclopropenone polymerizes ...
s and cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepare ...
diones can be converted to alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
s by elimination of one or two molecules of CO, respectively.
Biochemistry
Carbon monoxide is released in the degradation (catabolism) of heme by the action of O2, NADPH and the enzymeheme oxygenase
Heme oxygenase, or haem oxygenase, (HMOX, commonly abbreviated as HO) is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous ion, and carbon monoxide.
There are many heme degrading enzymes in nature. In general, on ...
:Ryter, S. W.; Tyrrell, R. M., "The Heme Synthesis and Degradation Pathways: Role in Oxidant Sensitivity: Heme Oxygenase Has Both Pro- and Antioxidant Properties", Free Radical Biology and Medicine 2000, volume 28, pages 289-309.
:
Inorganic and organometallic synthesis
Many metal carbonyls are prepared via decarbonylation reactions. The CO ligand inVaska's complex
Vaska's complex is the trivial name for the chemical compound ''trans''-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO) (C6H5)3sub>2. This square planar diamagnetic organometallic complex consists of a central iri ...
arises by the decarbonylation of DMF:
:IrCl3(H2O)3 + 3 P(C6H5)3 + HCON(CH3)2 + C6H5NH2 → IrCl(CO) (C6H5)3sub>2 + CH3)2NH2l + OP(C6H5)3 + 6H5NH3l + 2 H2O
The conversion of Fe(CO)5 and Mo(CO)6 to their many derivatives often involves decarbonylation. Here decarbonylation accompanies the preparation of Cyclopentadienyliron dicarbonyl dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crysta ...
:
:2 Fe(CO)5 + C10H12 → (''η''5-C5H5)2Fe2(CO)4 + 6 CO + H2
Decarbonylation can be induced photochemically as well as using reagents such as trimethylamine ''N''-oxide:
:Me3NO + L + Fe(CO)5 → Me3N + CO2 + LFe(CO)4
References
{{Reflist Chemical reactions Carbon monoxide