Debrancher Enzyme on:
[Wikipedia]
[Google]
[Amazon]
 A debranching enzyme is a molecule that helps facilitate the
A debranching enzyme is a molecule that helps facilitate the
GeneReviews/NCBI/NIH/UW entry on Glycogen Storage Disease Type III
OMIM entries on Glycogen Storage Disease Type III
* {{DEFAULTSORT:Glycogen Debranching Enzyme EC 2.4.1 EC 3.2.1
 A debranching enzyme is a molecule that helps facilitate the
A debranching enzyme is a molecule that helps facilitate the breakdown
Breakdown may refer to:
Breaking down
*Breakdown (vehicle), failure of a motor vehicle in such a way that it cannot be operated
*Chemical decomposition, also called chemical breakdown, the breakdown of a substance into simpler components
*Decompo ...
of glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
, which serves as a store of glucose in the body, through glucosyltransferase and glucosidase activity. Together with phosphorylases, debranching enzymes mobilize glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
reserves from glycogen deposits in the muscles and liver. This constitutes a major source of energy reserves in most organisms. Glycogen breakdown is highly regulated in the body, especially in the liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
, by various hormones including insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism o ...
and glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a Glucagon (medicati ...
, to maintain a homeostatic balance of blood-glucose levels. When glycogen breakdown is compromised by mutations in the glycogen debranching enzyme, metabolic diseases such as Glycogen storage disease type III
Glycogen storage disease type III (GSD III) is an autosomal recessive metabolic disorder and inborn error of metabolism (specifically of Inborn errors of carbohydrate metabolism, carbohydrates) characterized by a deficiency in glycogen debranching ...
can result.
Glucosyltransferase and glucosidase are performed by a single enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
in mammals, yeast, and some bacteria, but by two distinct enzymes in ''E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' and other bacteria, complicating nomenclature. Proteins that catalyze both functions are referred to as glycogen debranching enzymes (GDEs). When glucosyltransferase and glucosidase are catalyzed by distinct enzymes, "glycogen debranching enzyme" usually refers to the glucosidase enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
. In some literature, an enzyme capable only of glucosidase is referred to as a "debranching enzyme".
Function
Together with phosphorylase, glycogen debranching enzymes function inglycogen breakdown
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
Mechanism
The ...
and glucose mobilization. When phosphorylase has digested a glycogen branch down to four glucose residues, it will not remove further residues. Glycogen debranching enzymes assist phosphorylase, the primary enzyme involved in glycogen breakdown
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
Mechanism
The ...
, in the mobilization of glycogen stores. Phosphorylase can only cleave α-1,4- glycosidic bond between adjacent glucose molecules in glycogen but branches also exist as α-1,6 linkages. When phosphorylase reaches four residues from a branching point it stops cleaving; because 1 in 10 residues is branched, cleavage by phosphorylase alone would not be sufficient in mobilizing glycogen stores. Before phosphorylase can resume catabolism, debranching enzymes perform two functions:
* 4-α-D-glucanotransferase (), or glucosyltransferase Glucosyltransferases are a type of glycosyltransferase that enable the transfer of glucose.
Examples include:
* glycogen synthase
* glycogen phosphorylase
Glycogen phosphorylase is one of the phosphorylase enzymes (). Glycogen phosphorylase cat ...
, transfers three glucose residues from the four-residue glycogen branch to a nearby branch. This exposes a single glucose residue joined to the glucose chain through an α -1,6 glycosidic linkage
* Amylo-α-1,6-glucosidase (), or glucosidase, cleaves the remaining alpha-1,6 linkage, producing glucose and a linear chain of glycogen. The mechanism by which the glucosidase cleaves the α -1,6-linkage is not fully known because the amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
in the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
have not yet been identified. It is thought to proceed through a two step acid base assistance type mechanism, with an oxocarbenium
An oxocarbenium ion (or oxacarbenium ion) is a chemical species characterized by a central sp2-hybridized carbon, an oxygen substituent, and an overall positive charge that is delocalized between the central carbon and oxygen atoms. An oxocarbenium ...
ion intermediate, and retention of configuration in glucose. This is a common method through which to cleave bonds, with an acid below the site of hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
to lend a proton and a base above to deprotinate a water which can then act as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. These acids and bases are amino acid side chains in the active site of the enzyme. A scheme for the mechanism is shown in the figure below.
Thus the debranching enzymes, transferase and α-1,6- glucosidase converts the branched glycogen structure into a linear one, paving the way for further cleavage by phosphorylase.
Structure and activity
Two enzymes
In ''E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' and other bacteria, glucosyltransferase and glucosidase functions are performed by two distinct enzymes. In ''E. coli'', Glucose transfer is performed by 4-alpha-glucanotransferase, a 78.5 kDa protein coded for by the gene malQ. A second protein, referred to as debranching enzyme, performs α-1,6-glucose cleavage. This enzyme has a molecular mass of 73.6 kDa, and is coded for by the gene glgX. Activity of the two enzymes is not always necessarily coupled. In ''E. coli'' glgX selectively catalyzes the cleavage of 4-subunit branches, without the action of glucanotransferase. The product of this cleavage, maltotetraose
Maltodextrin is a polysaccharide that is used as a food ingredient. It is produced from vegetable starch by partial hydrolysis and is usually found as a white hygroscopic spray-dried powder. Maltodextrin is easily digestible, being absorbed as ...
, is further degraded by maltodextrin phosphorylase.
''E. coli'' GlgX is structurally similar to the protein isoamylase
Isoamylase (, ''debranching enzyme'', ''glycogen alpha-1,6-glucanohydrolase'') is an enzyme with systematic name ''glycogen 6-alpha-D-glucanohydrolase''. This enzyme catalyses the following chemical reaction
: Hydrolysis of (1->6)-alpha-D-glucosi ...
. The monomeric protein contains a central domain in which eight parallel beta-strands are surrounded by eight parallel alpha strands. Notable within this structure is a groove 26 angstroms long and 9 angstroms wide, containing aromatic residues that are thought to stabilize a four-glucose branch before cleavage.
The glycogen-degrading enzyme of the archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
'' Sulfolobus solfataricus'', treX, provides an interesting example of using a single active site for two activities: amylosidase and glucanotransferase activities. TreX is structurally similar to glgX, and has a mass of 80kD and one active site. Unlike either glgX, however, treX exists as a dimer and tetramer in solution. TreX's oligomeric form seems to play a significant role in altering both enzyme shape and function. Dimerization is thought to stabilize a "flexible loop" located close to the active site. This may be key to explaining why treX (and not glgX) shows glucosyltransferase activity. As a tetramer, the catalytic efficiency of treX is increased fourfold over its dimeric form.
One enzyme with two catalytic sites
In mammals andyeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
, a single enzyme performs both debranching functions. The human glycogen debranching enzyme (gene: AGL) is a monomer with a molecular weight of 175 kDa. It has been shown that the two catalytic actions of AGL can function independently of each other, demonstrating that multiple active sites are present. This idea has been reinforced with inhibitors of the active site, such as polyhydroxyamine, which were found to inhibit glucosidase activity while transferase activity was not measurably changed. Glycogen debranching enzyme is the only known eukaryotic enzyme that contains multiple catalytic sites and is active as a monomer.
Some studies have shown that the C-terminal half of yeast GDE is associated with glucosidase activity, while the N-terminal half is associated with glucosyltransferase activity. In addition to these two active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
s, AGL appears to contain a third active site that allows it to bind to a glycogen polymer. It is thought to bind to six glucose molecules of the chain as well as the branched glucose, thus corresponding to 7 subunits within the active site, as shown in the figure below.
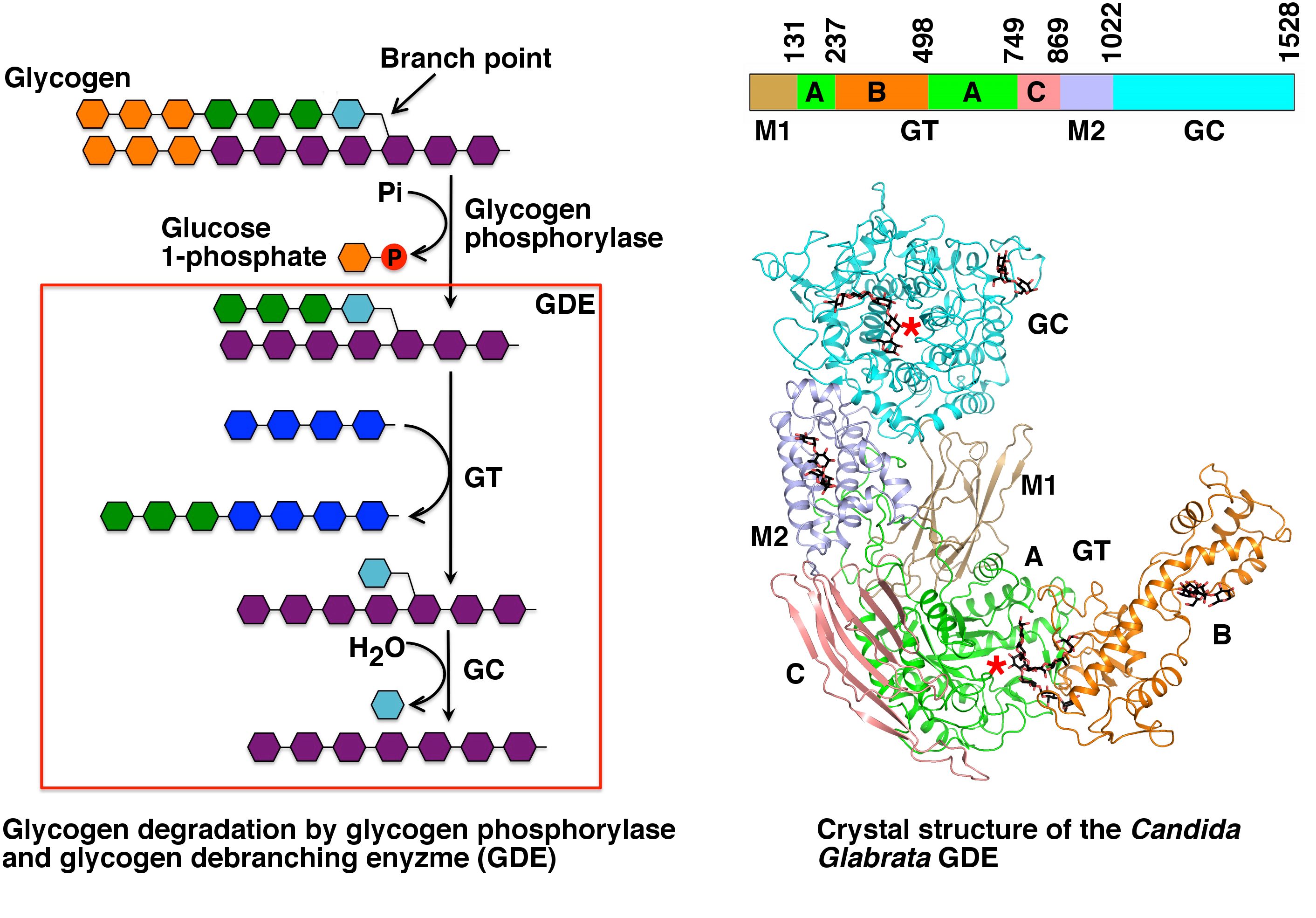
The structure of the ''Candida glabrata'' GDE has been reported. The structure revealed that distinct domains in GDE encode the glucanotransferase and glucosidase activities. Their catalyses are similar to that of alpha-amylase and glucoamylase, respectively. Their active sites are selective towards the respective substrates, ensuring proper activation of GDE. Besides the active sites GDE have additional binding sites for glycogen, which are important for its recruitment to glycogen. Mapping the disease-causing mutations onto the GDE structure provided insights into glycogen storage disease type III.
Genetic location
The official name for the gene is "amylo- α- 1,6- glucosidase, 4- α- glucanotransferase", with the official symbol AGL. AGL is an autosomal gene found on chromosome lp21. The AGL gene provides instructions for making several different versions, known as isoforms, of the glycogen debranching enzyme. These isoforms vary by size and are expressed in different tissues, such as liver and muscle. This gene has been studied in great detail, because mutation at this gene is the cause of Glycogen Storage Disease Type III. The gene is 85 kb long, has 35exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequen ...
s and encodes for a 7.0 kb mRNA. Translation of the gene begins at exon 3,which encodes for the first 27 amino acids of the AGL gene, because the first two exons (68kb) contain the 5' untranslated region. Exons 4-35 encode the remaining 1505 amino acids of the AGL gene.
Studies produced by the department of pediatrics at Duke University suggest that the human AGL gene contains at minimum 2 promotor regions, sites where the transcription of the gene begins, that result in differential expression of isoform, different forms of the same protein, mRNAs in a manner that is specific for different tissues.
Clinical significance
When GDE activity is compromised, the body cannot effectively release stored glycogen, type III Glycogen Storage Disease (debrancher deficiency), an autosomal recessive disorder, can result. In GSD III glycogen breakdown is incomplete and there is accumulation of abnormal glycogen with short outer branches. Most patients exhibit GDE defiency in both liver and muscle (Type IIIa), although 15% of patients have retained GDE in muscle while having it absent from the liver (Type IIIb). Depending onmutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
location, different mutations in the AGL gene can affect different isoforms of the gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. The ...
. For example, mutations that occur on exon 3, affect the form which affect the isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isof ...
that is primarily expressed in the liver; this would lead to GSD type III.
These different manifestation produce varied symptoms, which can be nearly indistinguishable from Type I GSD, including hepatomegaly, hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose belo ...
in children, short stature, myopathy
In medicine, myopathy is a disease of the muscle in which the muscle fibers do not function properly. This results in muscular weakness. ''Myopathy'' means muscle disease (Greek : myo- ''muscle'' + patheia '' -pathy'' : ''suffering''). This meani ...
, and cardiomyopathy
Cardiomyopathy is a group of diseases that affect the heart muscle. Early on there may be few or no symptoms. As the disease worsens, shortness of breath, feeling tired, and swelling of the legs may occur, due to the onset of heart failure. A ...
. Type IIIa patients often exhibit symptoms related to liver disease and progressive muscle involvement, with variations caused by age of onset, rate of disease progression and severity. Patients with Type IIIb generally symptoms related to liver disease. Type III patients be distinguished by elevated liver enzymes, with normal uric acid and blood lactate levels, differing from other forms of GSD. In patients with muscle involvement, Type IIIa, the muscle weakness becomes predominant into adulthood and can lead to ventricular hypertrophy and distal muscle wasting.
References
External links
GeneReviews/NCBI/NIH/UW entry on Glycogen Storage Disease Type III
OMIM entries on Glycogen Storage Disease Type III
* {{DEFAULTSORT:Glycogen Debranching Enzyme EC 2.4.1 EC 3.2.1