Curing (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
Curing is a chemical process employed in polymer chemistry and
 During the curing process, single monomers and oligomers, mixed with or without a curing agent, react to form a tridimensional polymeric network.
In the very first part of the reaction branches of molecules with various architectures are formed, and their
During the curing process, single monomers and oligomers, mixed with or without a curing agent, react to form a tridimensional polymeric network.
In the very first part of the reaction branches of molecules with various architectures are formed, and their
 Figure 3: Simplified chemical reactions associated with curing of a drying oil. In the first step, the diene undergoes autoxidation to give a hydroperoxide. In the second step, the hydroperoxide combines with another unsaturated side chain to generate a crosslink.
Epoxy, Epoxy resins are typically cured by the use of additives, often called hardeners. Polyamines are often used. The amine groups ring-open the epoxide rings.
In rubber, the curing is also induced by the addition of a crosslinker. The resulting process is called
Figure 3: Simplified chemical reactions associated with curing of a drying oil. In the first step, the diene undergoes autoxidation to give a hydroperoxide. In the second step, the hydroperoxide combines with another unsaturated side chain to generate a crosslink.
Epoxy, Epoxy resins are typically cured by the use of additives, often called hardeners. Polyamines are often used. The amine groups ring-open the epoxide rings.
In rubber, the curing is also induced by the addition of a crosslinker. The resulting process is called
 A simple way to monitor the change in viscosity, and thus, the extent of the reaction, in a curing process is to measure the variation of the elastic modulus.
To measure the elastic modulus of a system during curing, a
A simple way to monitor the change in viscosity, and thus, the extent of the reaction, in a curing process is to measure the variation of the elastic modulus.
To measure the elastic modulus of a system during curing, a
process engineering
Process engineering is the understanding and application of the fundamental principles and laws of nature that allow humans to transform raw material and energy into products that are useful to society, at an industrial level. By taking advantage ...
that produces the toughening or hardening of a polymer material by cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing of polymer chains. Even if it is strongly associated with the production of thermosetting polymer
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
s, the term "curing" can be used for all the processes where a solid product is obtained from a liquid solution, such as with PVC plastisol
A plastisol is a colloidal dispension of small polymer particles, usually polyvinyl chloride (PVC), in a liquid plasticizer. When heated to around , the plastic particles absorb the plasticizer, causing them to swell and fuse together forming a ...
s.
Curing process
molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
increases in time with the extent of the reaction until the network size is equal to the size of the system. The system has lost its solubility and its viscosity tends to infinite. The remaining molecules start to coexist with the macroscopic network until they react with the network creating other crosslink
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
s. The crosslink density increases until the system reaches the end of the chemical reaction.
Curing can be induced by heat, radiation, electron beams, or chemical additives. To quote from IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
: curing "might or might not require mixing with a chemical curing agent." Thus, two broad classes are (i) curing induced by chemical additives (also called curing agents, hardeners) and (ii) curing in the absence of additives. An intermediate case involves a mixture of resin and additives that requires external stimulus (light, heat, radiation) to induce curing.
The curing methodology depends on the resin and the application. Particular attention is paid to the shrinkage induced by the curing. Usually small values of shrinkage (2-3%) are desirable.
Curing induced by additives
 Figure 3: Simplified chemical reactions associated with curing of a drying oil. In the first step, the diene undergoes autoxidation to give a hydroperoxide. In the second step, the hydroperoxide combines with another unsaturated side chain to generate a crosslink.
Epoxy, Epoxy resins are typically cured by the use of additives, often called hardeners. Polyamines are often used. The amine groups ring-open the epoxide rings.
In rubber, the curing is also induced by the addition of a crosslinker. The resulting process is called
Figure 3: Simplified chemical reactions associated with curing of a drying oil. In the first step, the diene undergoes autoxidation to give a hydroperoxide. In the second step, the hydroperoxide combines with another unsaturated side chain to generate a crosslink.
Epoxy, Epoxy resins are typically cured by the use of additives, often called hardeners. Polyamines are often used. The amine groups ring-open the epoxide rings.
In rubber, the curing is also induced by the addition of a crosslinker. The resulting process is called sulfur vulcanization
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cr ...
. Sulfur breaks down to form polysulfide cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
s (bridges) between sections of the polymer chain
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
s. The degree of crosslinking determines the rigidity and durability, as well as other properties of the material.
Paints and varnishes commonly contain oil drying agent An oil drying agent, also known as siccative, is a coordination compound that accelerates (catalyzes) the hardening of drying oils, often as they are used in oil-based paints. This so-called "drying" (actually a chemical reaction that produces an or ...
s, usually metallic soap
A metallic soap is a metallic salt of a fatty acid. Theoretically, soaps can be made of any metal, although not all enjoy practical uses. Varying the metal can strongly affect the properties of the compound, particularly its solubility.
Alkali, ...
s that catalyze cross-linking of the unsaturated drying oils that largely comprise them. When paint is described as "drying" it is in fact hardening by crosslinking. Oxygen atoms serve as the crosslinks, analogous to the role played by sulfur in the vulcanization of rubber.
Curing without additives
In the case of concrete, curing entails the formation of silicate crosslinks. The process is not induced by additives. In many cases, the resin is provided as a solution or mixture with a thermally-activated catalyst, which induces crosslinking but only upon heating. For example, some acrylate-based resins are formulated withdibenzoyl peroxide
Benzoyl peroxide is a chemical compound (specifically, an organic peroxide) with structural formula , often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (, Bz) groups connected by a peroxide ( ...
. Upon heating the mixture, the peroxide converts to a free radical, which adds to an acrylate, initiating crosslinking.
Some organic resins are cured with heat. As heat is applied, the viscosity of the resin drops before the onset of crosslink
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing, whereupon it increases as the constituent oligomers interconnect. This process continues until a tridimensional network of oligomer chains is created – this stage is termed gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched p ...
. In terms of processability of the resin this marks an important stage: before gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched p ...
the system is relatively mobile, after it the mobility is very limited, the micro-structure of the resin and the composite material is fixed and severe diffusion limitations to further cure are created. Thus, in order to achieve vitrification in the resin, it is usually necessary to increase the process temperature after gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched p ...
.
When catalysts are activated by ultraviolet radiation, the process is called UV cure.Gregory T. Carroll, Nicholas J. Turro and Jeffrey T. Koberstein (2010) ''Patterning Dewetting in Thin Polymer Films by Spatially Directed Photocrosslinking'' Journal of Colloid and Interface Science, Vol. 351, pp 556-560
Monitoring methods
Cure monitoring is, for example, an essential component for the control of the manufacturing process of composite materials. The material, initially liquid, at the end of the process will be solid: viscosity is the most important property that changes during the process. Cure monitoring relies on monitoring various physical or chemical properties.Rheological analysis
 A simple way to monitor the change in viscosity, and thus, the extent of the reaction, in a curing process is to measure the variation of the elastic modulus.
To measure the elastic modulus of a system during curing, a
A simple way to monitor the change in viscosity, and thus, the extent of the reaction, in a curing process is to measure the variation of the elastic modulus.
To measure the elastic modulus of a system during curing, a rheometer
A rheometer is a laboratory device used to measure the way in which a dense fluid (a liquid, suspension or slurry) flows in response to applied forces. It is used for those fluids which cannot be defined by a single value of viscosity and th ...
can be used. With dynamic mechanical analysis
Dynamic mechanical analysis (abbreviated DMA) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers. A sinusoidal stress is applied and the strain in the material is measured ...
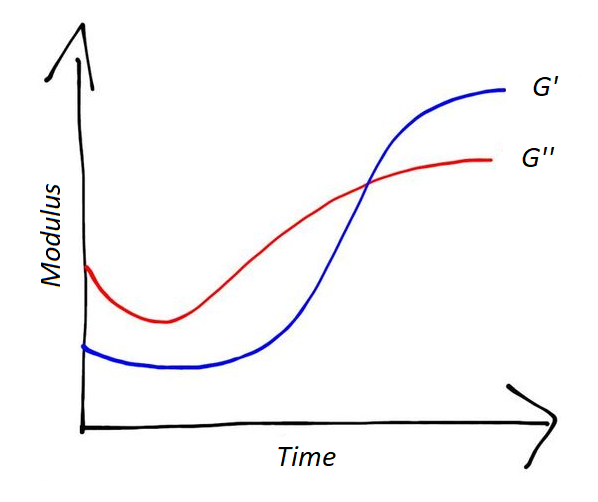
, the storage modulus (G’) and the loss modulus (G’’) can be measured. The variation of G' and G" in time can indicate the extent of the curing reaction.
As shown in Figure 4, after an "induction time”, G' and G" start to increase, with an abrupt change in slope. At a certain point they cross each other; afterwards, the rates of G' and G" decrease, and the moduli tend to a plateau. When they reach the plateau the reaction is concluded.
When the system is liquid, the storage modulus is very low: the system behaves like a liquid. Then the reaction continues and the system starts to react more like a solid: the storage modulus increases.
The degree of curing, , can be defined as follow:
The degree of curing starts from zero (at the beginning of the reaction) and grows until one (the end of the reaction). The slope of the curve changes with time and has his maximum about at half of the reaction.
Thermal analysis
If the reactions occurring during crosslinking are exothermic, the crosslinking rate can be related to the heat released during the process. Higher is the number ofbond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
s created, higher is the heat released in the reaction. At the end of the reaction, no more heat will be released. To measure the heat flow differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and ref ...
can be used.
Assuming that each bond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
formed during the crosslink
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing releases the same amount of energy, the degree of curing, , can be defined as follows:
where is the heat released up to a certain time , is the instantaneous rate of heat and is the total amount of heat released in , when the reaction finishes.
Also in this case the degree of curing goes from zero (no bonds created) to one (no more reactions occur) with a slope that changes in time and has its maximum about at half of the reaction.
Dielectrometric analysis
Conventional dielectrometry is carried out typically in a parallel plate configuration of the dielectric sensor (capacitance probe Capacitance sensors (or Dielectric sensors) use capacitance to measure the dielectric permittivity of a surrounding medium.
The configuration is like the neutron probe where an access tube made of PVC is installed in the soil; probes can also be mo ...
) and has the capability of monitoring the resin cure throughout the entire cycle, from the liquid to the rubber to the solid state. It is capable of monitoring phase separation in complex resin blends curing also within a fibrous perform. The same attributes belong to the more recent development of the dielectric technique, namely microdielectrometry.
Several versions of dielectric sensors are available commercially. The most suitable format for use in cure monitoring applications are the flat interdigital capacitive structures bearing a sensing grid on their surface. Depending on their design (specifically those on durable substrates) they have some reusability, while flexible substrate sensors can be used also in the bulk of the resin systems as embedded sensors.
Spectroscopic analysis
The curing process can be monitored by measuring changes in various parameters: *the concentration of specific reactive resin species using spectroscopic methods such as FTIR &Raman
Raman may refer to:
People
* Raman (name)
* C. V. Raman (1888–1970), Indian Nobel Prize-winning physicist
Places
* Raman, Punjab (India)
* Raman, Rawalpindi, Pakistan
* Raman District, Yala Province, Thailand
** Raman Railway Station
* R ...
;
*the refractive index or fluorescence of the resin (optical property);
*the internal resin strain (mechanical property) with the use of Fiber Bragg grating (FBG) sensors.
Ultrasonic analysis
Ultrasonic
Ultrasound is sound waves with frequencies higher than the upper audible limit of human hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hear it. This limit varies fr ...
cure monitoring methods are based on the relationships between changes in the characteristics of propagating ultrasound and the real-time mechanical properties of a component, by measuring:
*ultrasonic time of flight, both in through-transmission and pulse-echo modes;
*natural frequency using impact excitation and laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The firs ...
-induced surface acoustic wave
Acoustic waves are a type of energy propagation through a medium by means of adiabatic loading and unloading. Important quantities for describing acoustic waves are acoustic pressure, particle velocity, particle displacement and acoustic intensi ...
velocity measurement.
See also
*Vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include ...
* Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
References
* * *I.Partridge and G.Maistros, ‘Dielectric Cure Monitoring for Process Control’, Chapter 17, Vol. 5, Encyclopaedia of Composite Materials (2001), Elsevier Science, London, page 413 *P.Ciriscioli and G.Springer, ‘Smart Autoclave cure in Composites’, (1991), Technomic Publishing, Lancaster, PA. {{Authority control Polymer chemistry Chemical processes