Crystal Growth on:
[Wikipedia]
[Google]
[Amazon]
A
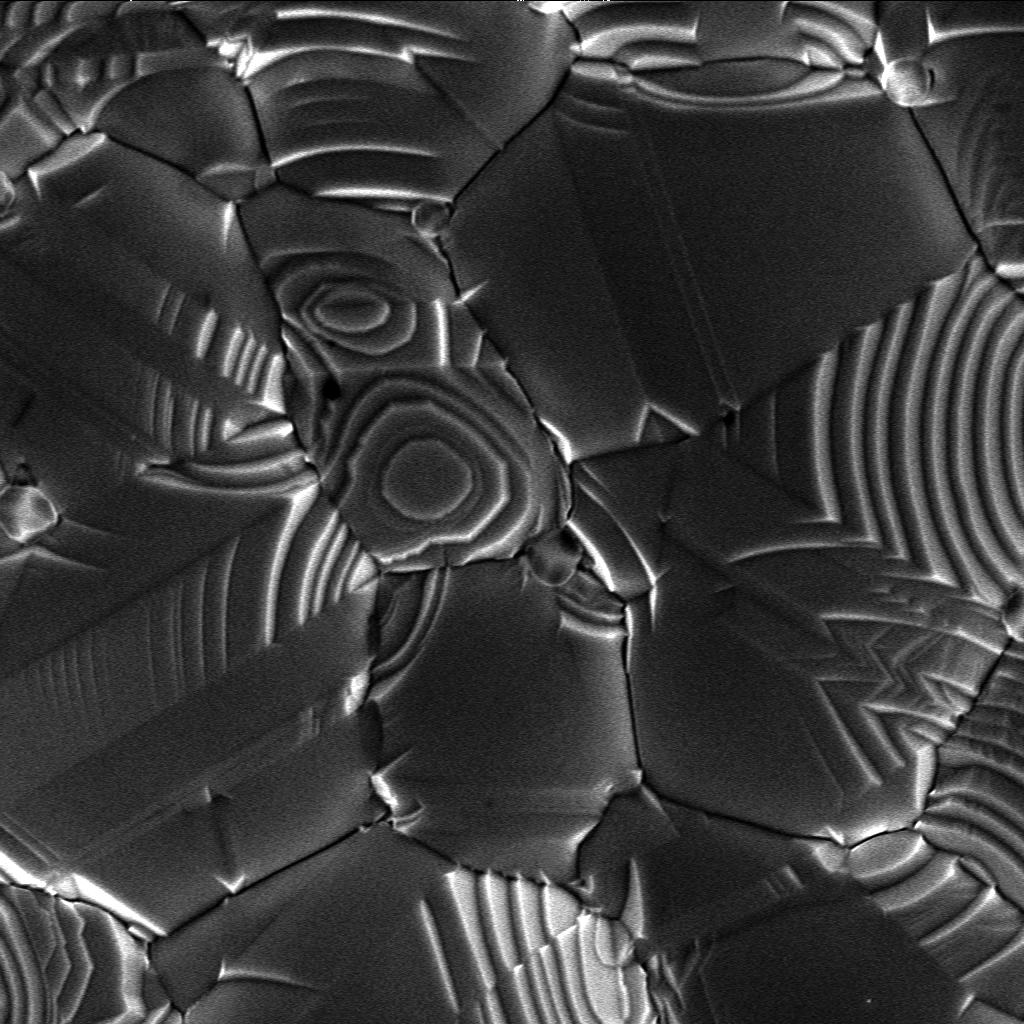 Nucleation can be either
Nucleation can be either
 The interface between a crystal and its vapor can be molecularly sharp at temperatures well below the melting point. An ideal crystalline surface grows by the spreading of single layers, or equivalently, by the lateral advance of the growth steps bounding the layers. For perceptible growth rates, this mechanism requires a finite driving force (or degree of supercooling) in order to lower the nucleation barrier sufficiently for nucleation to occur by means of thermal fluctuations. In the theory of crystal growth from the melt, Burton and Cabrera have distinguished between two major mechanisms:
The interface between a crystal and its vapor can be molecularly sharp at temperatures well below the melting point. An ideal crystalline surface grows by the spreading of single layers, or equivalently, by the lateral advance of the growth steps bounding the layers. For perceptible growth rates, this mechanism requires a finite driving force (or degree of supercooling) in order to lower the nucleation barrier sufficiently for nucleation to occur by means of thermal fluctuations. In the theory of crystal growth from the melt, Burton and Cabrera have distinguished between two major mechanisms:
 It is generally believed that the mechanical and other properties of the crystal are also pertinent to the subject matter, and that crystal morphology provides the missing link between growth kinetics and physical properties. The necessary thermodynamic apparatus was provided by
It is generally believed that the mechanical and other properties of the crystal are also pertinent to the subject matter, and that crystal morphology provides the missing link between growth kinetics and physical properties. The necessary thermodynamic apparatus was provided by

 Very commonly when the supersaturation (or degree of supercooling) is high, and sometimes even when it is not high, growth kinetics may be diffusion-controlled. Under such conditions, the polyhedral crystal form will be unstable, it will sprout protrusions at its corners and edges where the degree of supersaturation is at its highest level. The tips of these protrusions will clearly be the points of highest supersaturation. It is generally believed that the protrusion will become longer (and thinner at the tip) until the effect of interfacial free energy in raising the chemical potential slows the tip growth and maintains a constant value for the tip thickness.
In the subsequent tip-thickening process, there should be a corresponding instability of shape. Minor bumps or "bulges" should be exaggerated — and develop into rapidly growing side branches. In such an unstable (or metastable) situation, minor degrees of anisotropy should be sufficient to determine directions of significant branching and growth. The most appealing aspect of this argument, of course, is that it yields the primary morphological features of
Very commonly when the supersaturation (or degree of supercooling) is high, and sometimes even when it is not high, growth kinetics may be diffusion-controlled. Under such conditions, the polyhedral crystal form will be unstable, it will sprout protrusions at its corners and edges where the degree of supersaturation is at its highest level. The tips of these protrusions will clearly be the points of highest supersaturation. It is generally believed that the protrusion will become longer (and thinner at the tip) until the effect of interfacial free energy in raising the chemical potential slows the tip growth and maintains a constant value for the tip thickness.
In the subsequent tip-thickening process, there should be a corresponding instability of shape. Minor bumps or "bulges" should be exaggerated — and develop into rapidly growing side branches. In such an unstable (or metastable) situation, minor degrees of anisotropy should be sufficient to determine directions of significant branching and growth. The most appealing aspect of this argument, of course, is that it yields the primary morphological features of
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
is a solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structur ...
material
Material is a substance or mixture of substances that constitutes an object. Materials can be pure or impure, living or non-living matter. Materials can be classified on the basis of their physical and chemical properties, or on their geolo ...
whose constituent atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
s, molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the addition of new atoms, ions, or polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
, unless a "seed" crystal, purposely added to start the growth, was already present.
The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space
Space is the boundless three-dimensional extent in which objects and events have relative position and direction. In classical physics, physical space is often conceived in three linear dimensions, although modern physicists usually consi ...
relative to each other.
The crystalline state of matter is characterized by a distinct structural rigidity and very high resistance to deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied ...
and of the shear modulus of elasticity. This contrasts with most liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, ...
s or fluid
In physics, a fluid is a liquid, gas, or other material that continuously deforms (''flows'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are substances which cannot resist any shear ...
s, which have a low shear modulus, and typically exhibit the capacity for macroscopic viscous flow.
Overview
After successful formation of a stable nucleus, a growth stage ensues in which free particles (atoms or molecules) adsorb onto the nucleus and propagate its crystalline structure outwards from the nucleating site. This process is significantly faster than nucleation. The reason for such rapid growth is that real crystals contain dislocations and other defects, which act as a catalyst for the addition of particles to the existing crystalline structure. By contrast, perfect crystals (lacking defects) would grow exceedingly slowly. On the other hand, impurities can act as crystal growth inhibitors and can also modifycrystal habit
In mineralogy, crystal habit is the characteristic external shape of an individual crystal or crystal group. The habit of a crystal is dependent on its crystallographic form and growth conditions, which generally creates irregularities due to l ...
.
Nucleation
 Nucleation can be either
Nucleation can be either homogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
, without the influence of foreign particles, or heterogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
, with the influence of foreign particles. Generally, heterogeneous nucleation takes place more quickly since the foreign particles act as a scaffold for the crystal to grow on, thus eliminating the necessity of creating a new surface and the incipient surface energy requirements.
Heterogeneous nucleation can take place by several methods. Some of the most typical are small inclusions, or cuts, in the container the crystal is being grown on. This includes scratches on the sides and bottom of glassware. A common practice in crystal growing is to add a foreign substance, such as a string or a rock, to the solution, thereby providing nucleation sites for facilitating crystal growth and reducing the time to fully crystallize.
The number of nucleating sites can also be controlled in this manner. If a brand-new piece of glassware or a plastic container is used, crystals may not form because the container surface is too smooth to allow heterogeneous nucleation. On the other hand, a badly scratched container will result in many lines of small crystals. To achieve a moderate number of medium-sized crystals, a container which has a few scratches works best. Likewise, adding small previously made crystals, or seed crystals, to a crystal growing project will provide nucleating sites to the solution. The addition of only one seed crystal should result in a larger single crystal.
Mechanisms of growth
 The interface between a crystal and its vapor can be molecularly sharp at temperatures well below the melting point. An ideal crystalline surface grows by the spreading of single layers, or equivalently, by the lateral advance of the growth steps bounding the layers. For perceptible growth rates, this mechanism requires a finite driving force (or degree of supercooling) in order to lower the nucleation barrier sufficiently for nucleation to occur by means of thermal fluctuations. In the theory of crystal growth from the melt, Burton and Cabrera have distinguished between two major mechanisms:
The interface between a crystal and its vapor can be molecularly sharp at temperatures well below the melting point. An ideal crystalline surface grows by the spreading of single layers, or equivalently, by the lateral advance of the growth steps bounding the layers. For perceptible growth rates, this mechanism requires a finite driving force (or degree of supercooling) in order to lower the nucleation barrier sufficiently for nucleation to occur by means of thermal fluctuations. In the theory of crystal growth from the melt, Burton and Cabrera have distinguished between two major mechanisms:
Non-uniform lateral growth
The surface advances by the lateral motion of steps which are one interplanar spacing in height (or some integral multiple thereof). An element of surface undergoes no change and does not advance normal to itself except during the passage of a step, and then it advances by the step height. It is useful to consider the step as the transition between two adjacent regions of a surface which are parallel to each other and thus identical in configuration — displaced from each other by an integral number of lattice planes. Note here the distinct possibility of a step in a diffuse surface, even though the step height would be much smaller than the thickness of the diffuse surface.Uniform normal growth
The surface advances normal to itself without the necessity of a stepwise growth mechanism. This means that in the presence of a sufficient thermodynamic driving force, every element of surface is capable of a continuous change contributing to the advancement of the interface. For a sharp or discontinuous surface, this continuous change may be more or less uniform over large areas each successive new layer. For a more diffuse surface, a continuous growth mechanism may require change over several successive layers simultaneously. Non-uniform lateral growth is a geometrical motion of steps — as opposed to motion of the entire surface normal to itself. Alternatively, uniform normal growth is based on the time sequence of an element of surface. In this mode, there is no motion or change except when a step passes via a continual change. The prediction of which mechanism will be operative under any set of given conditions is fundamental to the understanding of crystal growth. Two criteria have been used to make this prediction: Whether or not the surface is ''diffuse'': a diffuse surface is one in which the change from one phase to another is continuous, occurring over several atomic planes. This is in contrast to a sharp surface for which the major change in property (e.g. density or composition) is discontinuous, and is generally confined to a depth of one interplanar distance. Whether or not the surface is ''singular'': a singular surface is one in which the surface tension as a function of orientation has a pointed minimum. Growth of singular surfaces is known to requires steps, whereas it is generally held that non-singular surfaces can continuously advance normal to themselves.Driving force
Consider next the necessary requirements for the appearance of lateral growth. It is evident that the lateral growth mechanism will be found when any area in the surface can reach a metastable equilibrium in the presence of a driving force. It will then tend to remain in such an equilibrium configuration until the passage of a step. Afterward, the configuration will be identical except that each part of the step but will have advanced by the step height. If the surface cannot reach equilibrium in the presence of a driving force, then it will continue to advance without waiting for the lateral motion of steps. Thus, Cahn concluded that the distinguishing feature is the ability of the surface to reach an equilibrium state in the presence of the driving force. He also concluded that for every surface or interface in a crystalline medium, there exists a critical driving force, which, if exceeded, will enable the surface or interface to advance normal to itself, and, if not exceeded, will require the lateral growth mechanism. Thus, for sufficiently large driving forces, the interface can move uniformly without the benefit of either a heterogeneous nucleation or screw dislocation mechanism. What constitutes a sufficiently large driving force depends upon the diffuseness of the interface, so that for extremely diffuse interfaces, this critical driving force will be so small that any measurable driving force will exceed it. Alternatively, for sharp interfaces, the critical driving force will be very large, and most growth will occur by the lateral step mechanism. Note that in a typicalsolidification
Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liqu ...
or crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
process, the thermodynamic driving force is dictated by the degree of supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
.
Morphology
 It is generally believed that the mechanical and other properties of the crystal are also pertinent to the subject matter, and that crystal morphology provides the missing link between growth kinetics and physical properties. The necessary thermodynamic apparatus was provided by
It is generally believed that the mechanical and other properties of the crystal are also pertinent to the subject matter, and that crystal morphology provides the missing link between growth kinetics and physical properties. The necessary thermodynamic apparatus was provided by Josiah Willard Gibbs
Josiah Willard Gibbs (; February 11, 1839 – April 28, 1903) was an American scientist who made significant theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in t ...
' study of heterogeneous equilibrium. He provided a clear definition of surface energy, by which the concept of surface tension is made applicable to solids as well as liquids. He also appreciated that ''an anisotropic surface free energy implied a non-spherical equilibrium shape'', which should be thermodynamically defined as ''the shape which minimizes the total surface free energy''.
It may be instructional to note that whisker
Vibrissae (; singular: vibrissa; ), more generally called Whiskers, are a type of stiff, functional hair used by mammals to sense their environment. These hairs are finely specialised for this purpose, whereas other types of hair are coars ...
growth provides the link between the mechanical phenomenon of high strength in whiskers and the various growth mechanisms which are responsible for their fibrous morphologies. (Prior to the discovery of carbon nanotubes, single-crystal whiskers had the highest tensile strength of any materials known). Some mechanisms produce defect-free whiskers, while others may have single screw dislocations along the main axis of growth — producing high strength whiskers.
The mechanism behind whisker growth is not well understood, but seems to be encouraged by compressive mechanical stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
es including mechanically induced stresses, stresses induced by diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical ...
of different elements, and thermally induced stresses. Metal whiskers differ from metallic dendrite
Dendrites (from Greek δένδρον ''déndron'', "tree"), also dendrons, are branched protoplasmic extensions of a nerve cell that propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the ...
s in several respects. Dendrites are fern
A fern (Polypodiopsida or Polypodiophyta ) is a member of a group of vascular plants (plants with xylem and phloem) that reproduce via spores and have neither seeds nor flowers. The polypodiophytes include all living pteridophytes exce ...
-shaped like the branches of a tree, and grow across the surface of the metal. In contrast, whiskers are fibrous and project at a right angle to the surface of growth, or substrate.
Diffusion-control

 Very commonly when the supersaturation (or degree of supercooling) is high, and sometimes even when it is not high, growth kinetics may be diffusion-controlled. Under such conditions, the polyhedral crystal form will be unstable, it will sprout protrusions at its corners and edges where the degree of supersaturation is at its highest level. The tips of these protrusions will clearly be the points of highest supersaturation. It is generally believed that the protrusion will become longer (and thinner at the tip) until the effect of interfacial free energy in raising the chemical potential slows the tip growth and maintains a constant value for the tip thickness.
In the subsequent tip-thickening process, there should be a corresponding instability of shape. Minor bumps or "bulges" should be exaggerated — and develop into rapidly growing side branches. In such an unstable (or metastable) situation, minor degrees of anisotropy should be sufficient to determine directions of significant branching and growth. The most appealing aspect of this argument, of course, is that it yields the primary morphological features of
Very commonly when the supersaturation (or degree of supercooling) is high, and sometimes even when it is not high, growth kinetics may be diffusion-controlled. Under such conditions, the polyhedral crystal form will be unstable, it will sprout protrusions at its corners and edges where the degree of supersaturation is at its highest level. The tips of these protrusions will clearly be the points of highest supersaturation. It is generally believed that the protrusion will become longer (and thinner at the tip) until the effect of interfacial free energy in raising the chemical potential slows the tip growth and maintains a constant value for the tip thickness.
In the subsequent tip-thickening process, there should be a corresponding instability of shape. Minor bumps or "bulges" should be exaggerated — and develop into rapidly growing side branches. In such an unstable (or metastable) situation, minor degrees of anisotropy should be sufficient to determine directions of significant branching and growth. The most appealing aspect of this argument, of course, is that it yields the primary morphological features of dendritic
Dendrite derives from the Greek word "dendron" meaning ( "tree-like"), and may refer to:
Biology
*Dendrite, a branched projection of a neuron
* Dendrite (non-neuronal), branching projections of certain skin cells and immune cells
Physical
*Dendr ...
growth.
See also
* Abnormal grain growth *Chvorinov's rule
Chvorinov's rule is an applied physics relationship first expressed by Czech engineer Nicolas Chvorinov in 1940, that relates the solidification time for a simple casting to the volume and surface area of the casting. In simple terms the rule ...
*Cloud condensation nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This c ...
*Crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
* Czochralski process
*Dendrite (metal)
A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large co ...
* Diana's Tree
* Fractional crystallization
* Ice nucleus
* Laser-heated pedestal growth
* Manganese nodule
* Micro-pulling-down
* Monocrystalline whisker
* Protocrystalline
* Recrystallization (chemistry)
*Seed crystal
A seed crystal is a small piece of single crystal or polycrystal material from which a large crystal of typically the same material is grown in a laboratory. Used to replicate material, the use of seed crystal to promote growth avoids the other ...
* Single crystal
* Whisker (metallurgy)
Simulation
* Kinetic Monte Carlo surface growth methodReferences
{{DEFAULTSORT:Crystal Growth Crystallography Crystals Materials science Mineralogy Articles containing video clips