Covalent superconductors on:
[Wikipedia]
[Google]
[Amazon]
 Covalent superconductors are
Covalent superconductors are
L. Boeri, J. Kortus and O. K. Anderse
2-Type Superconductivity in Hole-Doped Diamond"">"Three-Dimensional MgB2-Type Superconductivity in Hole-Doped Diamond"
K.-W. Lee and W. E. Picket
"Superconductivity in Boron-Doped Diamond"
X. Blase, Ch. Adessi and D. Connetabl
"Role of the Dopant in the Superconductivity of Diamond"
E. Bustarret et al
"Dependence of the Superconducting Transition Temperature on the Doping Level in Single-Crystalline Diamond Films"
– free download The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
 The priority of many discoveries in science is vigorously disputed (see, e.g.,
The priority of many discoveries in science is vigorously disputed (see, e.g.,
International Workshop on superconductivity in Diamond and Related Materials
2005
International Workshop on Superconductivity in Diamond and Related Materials
2008
* ttp://www.nims.go.jp/NFM/paper1/SuperconductingDiamond/SuperconductingDiamond.html Some papers on superconducting diamond {{DEFAULTSORT:Covalent Superconductor Superconductivity Superconductors
 Covalent superconductors are
Covalent superconductors are superconducting
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
materials where the atoms are linked by covalent bonds. The first such material was boron-doped synthetic diamond
Lab-grown diamond (LGD; also called laboratory-grown, laboratory-created, man-made, artisan-created, artificial, synthetic, or cultured diamond) is diamond that is produced in a controlled technological process (in contrast to naturally formed ...
grown by the high-pressure high-temperature (HPHT) method.L. Boeri, J. Kortus and O. K. Anderse
2-Type Superconductivity in Hole-Doped Diamond"">"Three-Dimensional MgB2-Type Superconductivity in Hole-Doped Diamond"
K.-W. Lee and W. E. Picket
"Superconductivity in Boron-Doped Diamond"
X. Blase, Ch. Adessi and D. Connetabl
"Role of the Dopant in the Superconductivity of Diamond"
E. Bustarret et al
"Dependence of the Superconducting Transition Temperature on the Doping Level in Single-Crystalline Diamond Films"
– free download The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
History
 The priority of many discoveries in science is vigorously disputed (see, e.g.,
The priority of many discoveries in science is vigorously disputed (see, e.g., Nobel Prize controversies
Since the first award in 1901, conferment of the Nobel Prize has occasionally engendered criticismSumio Iijima
is a Japanese physicist and inventor, often cited as the inventor of carbon nanotubes. Although carbon nanotubes had been observed prior to his "invention", Iijima's 1991 paper generated unprecedented interest in the carbon nanostructures and ...
has "discovered" carbon nanotubes in 1991, many scientists have pointed out that carbon nanofibers were actually observed decades earlier. The same could be said about superconductivity in covalent semiconductors. Superconductivity in germanium and silicon-germanium was predicted theoretically as early as in the 1960s. Shortly after, superconductivity was experimentally detected in germanium telluride. In 1976, superconductivity with ''T''c = 3.5 K was observed experimentally in germanium implanted with copper ions; it was experimentally demonstrated that amorphization was essential for the superconductivity (in Ge), and the superconductivity was assigned to Ge itself, not copper.
Diamond
Superconductivity in diamond was achieved through heavy p-type doping by boron such that the individual doping atoms started interacting and formed an "impurity band". The superconductivity was of type-II with the critical temperature ''T''c = 4 K and critical magnetic field ''B''c = 4 T. Later, ''T''c ≈ 11 K has been achieved in homoepitaxial CVD films. Regarding the origin of superconductivity in diamond, three alternative theories were suggested: conventionalBCS theory
BCS theory or Bardeen–Cooper–Schrieffer theory (named after John Bardeen, Leon Cooper, and John Robert Schrieffer) is the first microscopic theory of superconductivity since Heike Kamerlingh Onnes's 1911 discovery. The theory describes sup ...
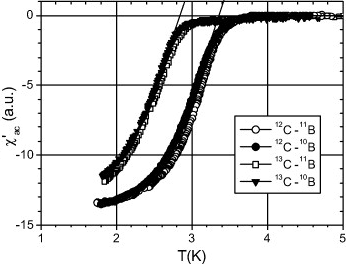
based on phonon-mediated pairing, correlated impurity band theory and spin-flip-driven pairing of holes weakly localized in the vicinity of the Fermi level. Experiments on diamonds enriched with 12C, 13C, 10B or 11B isotopes revealed a clear ''T''c shift, and its magnitude confirms the BCS mechanism of superconductivity in bulk polycrystalline diamond.
Carbon nanotubes
While there have been reports of intrinsic superconductivity incarbon nanotubes
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
, many other experiments found no evidence of superconductivity, and the validity of these results remains a subject of debate. Note, however, a crucial difference between nanotubes and diamond: Although nanotubes contain covalently bonded carbon atoms, they are closer in properties to graphite than diamond, and can be metallic without doping. Meanwhile, undoped diamond is an insulator.
Intercalated graphite
When metal atoms are inserted (intercalated) between the graphite planes, several superconductors are created with the following transition temperatures:Silicon
It was suggested that "Si and Ge, which also form in the diamond structure, may similarly exhibit superconductivity under the appropriate conditions", and indeed, discoveries of superconductivity in heavily boron doped Si (Si:B) and SiC:B have quickly followed. Similar to diamond, Si:B is type-II superconductor, but it has much smaller values of ''T''c = 0.4 K and ''B''c = 0.4 T. Superconductivity in Si:B was achieved by heavy doping (above 8 at.%), realized through a special non-equilibrium technique of gas immersion laser doping.Silicon carbide
Superconductivity inSiC
The Latin adverb ''sic'' (; "thus", "just as"; in full: , "thus was it written") inserted after a quoted word or passage indicates that the quoted matter has been transcribed or translated exactly as found in the source text, complete with any e ...
was achieved by heavy doping with boron or aluminum. Both the cubic (3C-SiC) and hexagonal (6H-SiC) phases are superconducting and show a very similar ''T''c of 1.5 K. A crucial difference is however observed for the magnetic field behavior between aluminum and boron doping: SiC:Al is type-II, same as Si:B. On the contrary, SiC:B is type-I. In attempt to explain this difference, it was noted that Si sites are more important than carbon sites for superconductivity in SiC. Whereas boron substitutes carbon in SiC, Al substitutes Si sites. Therefore, Al and B "see" different environment that might explain different properties of SiC:Al and SiC:B.
Hydrogen sulfide
At pressures above 90 GPa (gigapascal
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined as ...
), hydrogen sulfide becomes a metallic conductor of electricity. When cooled below a critical temperature its high-pressure phase exhibits superconductivity. The critical temperature increases with pressure, ranging from 23 K at 100 GPa to 150 K at 200 GPa. If hydrogen sulfide is pressurized at higher temperatures, then cooled, the critical temperature reaches , the highest accepted superconducting critical temperature as of 2015. By substituting a small part of sulfur with phosphorus and using even higher pressures, it has been predicted that it may be possible to raise the critical temperature to above and achieve room-temperature superconductivity.
See also
* * * * * * * * * * * * * *References
External links
International Workshop on superconductivity in Diamond and Related Materials
2005
International Workshop on Superconductivity in Diamond and Related Materials
2008
* ttp://www.nims.go.jp/NFM/paper1/SuperconductingDiamond/SuperconductingDiamond.html Some papers on superconducting diamond {{DEFAULTSORT:Covalent Superconductor Superconductivity Superconductors