Chiral Switch on:
[Wikipedia]
[Google]
[Amazon]
The word "chiral switch" was introduced by Agranat and Caner in 1999. Chiral switches are
chiral drugs
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mi ...
that are already approved as racemates but that have been re-developed as single enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
s. The term chiral switching has been coined to describe the development of single enantiomers from racemate drugs. For example, levofloxacin
Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori (in combination with other medications), ...
is a chiral switch of racemic ofloxacin
Ofloxacin is a quinolone antibiotic useful for the treatment of a number of bacterial infections. When taken Oral administration, by mouth or intravenous, injection into a vein, these include pneumonia, cellulitis, urinary tract infections, prost ...
. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coined eutomer and distomer. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.
In case of stereoselectivity in action only one of the components in the racemic mixture is truly active (eutomer). The other isomer, the distomer, should be regarded as impurity or isomeric ballast not contributing to the effects aimed at. It is well documented that the pharmacologically inactive isomer (distomer) may contribute to the toxic or adverse effects of the drugs. There is a wide spectrum of possibilities of distomer actions, many of which are confirmed experimentally. Sometimes the single enantiomer version lacks certain side-effects that the racemate exhibits. And where the two enantiomers are sufficiently different in pharmacological effects, it may be possible to get a patent on one or both isomers (for instance, as in the case of propoxyphene
Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive (cough suppressant) a ...
). The chiral twins of propoxyphene are separately sold by Eli Lilly and company. Dextropropoxyphene is an analgesic agent (Darvon) and levopropoxyphene an effective antitussive (Novrad). Interestingly the reversed trade names of the drugs, DARVON and NOVRAD, also reflect the chemical mirror-image relationship. A positive consequence of this redesigning approach is that it has given a new life to an old drug, minimizing or avoiding the undesirable side-effect profile. Whether to go in for a chiral switch is normally made on a case-by-case basis. A pragmatic solution could be in favor of a decision-tree approach, incorporating various factors such as pharmacodynamic
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (for ...
, pharmacokinetic
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered ...
, toxicological profile of the enantiomers, enantiomer-enantiomer interaction potential, safety, efficacy, risk-benefit ratio, chiral inversion, distomer liability, physicochemical properties, cost of separation and production, quality control criteria, marketing edge, etc.
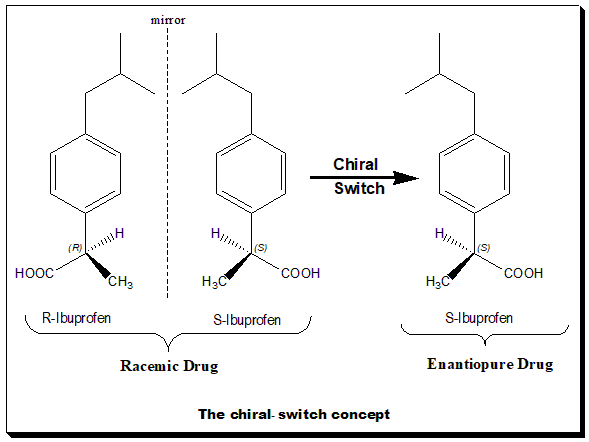
The chiral-switch concept
The chiral switch concept is illustrated in the diagram. This chiral switch is from (±)-ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arte ...
to (''S'')-(+)-ibuprofen (dexibuprofen
Dexibuprofen is a nonsteroidal anti-inflammatory drug (NSAID).
It is the active dextrorotatory enantiomer of ibuprofen. Most ibuprofen formulations contain a racemic mixture of both isomers.
Chemistry
Basically Dexibuprofen is a chiral ...
). The nonsteroidal anti-inflammatory drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of ...
(NSAID) ibuprofen was the first chiral drug of the NSAID class to be switched to the single-enantiomer version in 1994. The switch was done based on the fact that the (''S'')-ibuprofen, the eutomer, was over 100-fold more potent as an inhibitor of cycloxygenase-1 (COX-1) enzyme than (''R'')-ibuprofen. Moreover, ibuprofen, when administered as the racemate, the active (''R'')-enantiomer undergoes partial unidirectional chiral inversion Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temper ...
(approximately 60%) to the (S)-enantiomer. Therefore, the use of the single (S)-ibuprofen was expected to give faster onset of action at a lower dosage. Further, while choosing the chiral drug candidate for a chiral switch one should take a look at the chiral inversion Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temper ...
tendency of the molecule. For instance, thalidomide, the sedative drug, undergoes bidirectional chiral inversion Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temper ...
or racemization in biological systems. In such cases chiral switching efforts will be pointless.

Rationale for switching
There are several possible potential benefits to chiral switching or chiral specific drugs. These include: #An improved (less complex, more selective) pharmacodynamic profile #A highertherapeutic index
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes ...
(improved safety margin)
#Less complex pharmacokinetic profile, less complex drug interactions
#Less complex relationship between plasma concentration and effect
#More rational therapeutic drug monitoring
#Expose the patient to less body load and thus reduce metabolic/renal/hepatic drug load
The chiral switching approach has sometimes resulted in failures and disappointments.
Regulatory environment
The roles of regulatory agencies also continue to evolve with respect to the development of chiral switches. An interesting concept brought up in the FDA policy is that of "bridging studies". When a sponsor/innovator seeks to develop a single enantiomer from a racemic drug, the regulatory agencies demand them to conduct bridging studies. Bridging studies are tests (pharmacological and toxicological evaluations) to connect what is known about the already approved racemate and what is unknown about the single enantiomer under study, without going back to square one as for a completely new chemical entity. The intent of the bridging studies is to make sure that the companies are not scarifying some protective effect conferred by the other" isomer when they develop a chiral drug as single enantiomer rather than a racemate. "Bridging" procedure will help to reduce the number of studies required on the "new" enantiopure drug.Launched chiral switches
Chiral switch, a re-engineering approach, has enabled in the remarketing of a number of racemic drugs as chiral specific enantiomer products. Chiral switching strategy is the way most blockbuster drugs have entered the market as enantiopure drugs. But the alternate route is '' de novo'' (anew) synthesis of chiral specific drugs. The chiral switches may have the same, very similar, therapeutic indications as the original racemic drug. But, there are instances where new indications for the old drug have been reported. The table below gives a brief list of launched chiral switches.Failed/aborted chiral switches
The re-evaluation of single enantiomers not without problems. The chiral switches of fluoxetine and fenfluramine are classical examples. The development of (R )-fluoxetine was terminated after patients developed abnormal heart rhythms. The chiral switch of fenfluramine, dexfenfluramine was withdrawn from world marker due to pulmonary hypertension. The table below enumerates couple of chiral switches aborted or withdrawn due stereochemically engineered toxicity.
Evergreening
Evergreening is any of various legal, business, and technological strategies by which producers (often pharmaceutical companies) extend the lifetime of their patents that are about to expire in order to retain revenues from them. Often the practice ...
"Evergreening" refers to the various strategies whereby owners (innovators/sponsors) of pharmaceutical products use patent laws and minor drug modifications to extend their monopoly privileges on the drug. An enantiomer patent is another form of evergreening based on a chiral switch strategy. Single-enantiomer drugs represent more than 50% of the top-selling 100 drugs worldwide. There are some studies which go to suggest that drug companies employ chiral switching for life-cycle management/patent protection of the parent racemic drug and also as a marketing strategy. Pharmaceutical companies support evergreening practices. Some chiral switches are performed to re-start the patent clock for a medication without reducing side effects or improving efficacy. A high price can then continue to be charged for a medication. Examples include citalopram and escitalopram, and omeprazole and esomeprazole. In both these medications, proposed theoretical benefits were used to market the enantiopure drugs, without any clinical trials being conducted to provide evidence that the racemic drugs improved patient centered outcomes.
Chiral drug to metabolite switches
This idea, drug to metabolite switching, is an extension of the chiral switch concept. The purpose of the switching is to develop an active metabolite which will be devoid of the side-effects and have an improved therapeutic profile compared to the parent chiral drug. Some examples of chiral drug to metabolite switches, (those in the market and others under investigation) include terfenadine to fexofenadine, halofantrine to desbutylhalofantrine, and cisapride to norcisapride. A summary is presented in the table below.See also
*Chiral drugs
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mi ...
*Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
*Enantiopure drug An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules (proteins, sugars, etc.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind di ...
*Chiral inversion Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temper ...
References
{{Reflist Stereochemistry Enantiopure drugs