chalcone synthase on:
[Wikipedia]
[Google]
[Amazon]
Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase
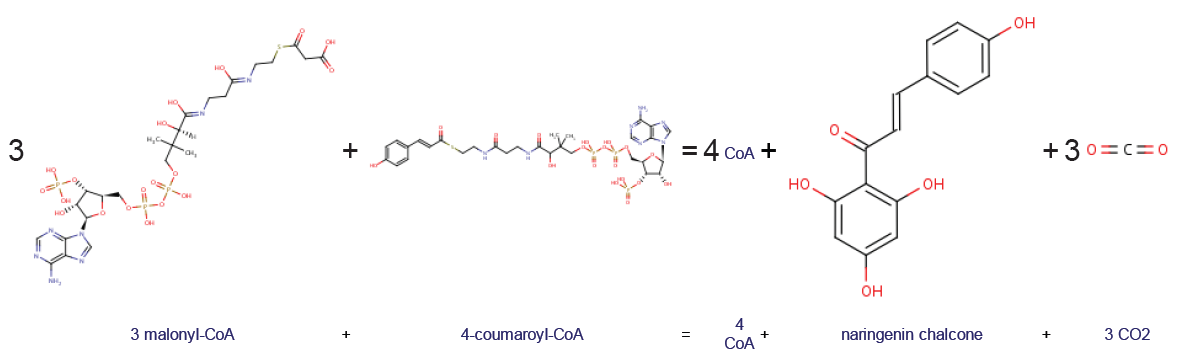 4-coumaroyl-CoA and three units of malonyl-CoA are converted into three molecules of
4-coumaroyl-CoA and three units of malonyl-CoA are converted into three molecules of
BRENDA entry
{{Portal bar, Biology, border=no EC 2.3.1 Chalconoids metabolism Enzymes of known structure Dihydrochalcones metabolism
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
biosynthesis.
The enzyme catalyzes the conversion of 4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include lignols (precursors to lignin and ligno ...
and malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and com ...
to naringenin chalcone
Naringenin chalcone is a common chalconoid (or chalcone, not to be confused with the compound chalcone). It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin ...
.
Function
CHS catalysis serves as the initial step for flavonoid biosynthesis. Flavonoids are important plant secondary metabolites that serve various functions in higher plants. These include pigmentation, UV protection, fertility, antifungal defense and the recruitment of nitrogen-fixing bacteria. CHS is believed to act as a central hub for the enzymes involved in the flavonoid pathway. Studies have shown that these enzymes interact via protein-protein interactions. Through FLIM FRET, it was shown that CHS interacts withchalcone isomerase
In enzymology, a chalcone isomerase () is an enzyme that catalyzes the chemical reaction
:a chalcone \rightleftharpoons a flavanone
Hence, this enzyme has one substrate, a chalcone, and one product, a flavanone.
This enzyme belongs to the fa ...
(CHI), a consecutive step enzyme, as well as other non-consecutive step enzymes flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), and flavonol synthase I.
Naringenin-chalcone synthase
Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, ...
uses malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and com ...
and 4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include lignols (precursors to lignin and ligno ...
to produce CoA
Coa may refer to:
Places
* Coa, County Fermanagh, a rural community in County Fermanagh, Northern Ireland
* Côa River, a tributary of the Douro, Portugal
** Battle of Coa, part of the Peninsular War period of the Napoleonic Wars
** Côa Valley ...
, naringenin chalcone
Naringenin chalcone is a common chalconoid (or chalcone, not to be confused with the compound chalcone). It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin ...
, and CO2.
Reaction
 4-coumaroyl-CoA and three units of malonyl-CoA are converted into three molecules of
4-coumaroyl-CoA and three units of malonyl-CoA are converted into three molecules of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, four molecules of coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a subs ...
and one unit of naringenin chalcone
Naringenin chalcone is a common chalconoid (or chalcone, not to be confused with the compound chalcone). It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin ...
.
Structure
Subunits
CHS exists as a homodimeric protein with each monomer approximately 42-45 kDa in size. Each monomer possesses a β-keto synthase (KS) activity that catalyzes the sequential head to tail incorporation of two-carbonacetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
units into a growing polyketide chain. CHS contains a five layer αβαβα core, a location of the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate ( binding site) ...
and dimerization
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic che ...
interface that is highly similar to thiolase-fold containing enzymes. The dimerization interface contains both hydrophobic and hydrophilic residues and is generally flat except for a pair of N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
helices that lay entwined across the top. Although the helices are not involved in reaction, they may contain intracellular localization signals as in yeast thiolase. They may also undergo a conformational change to participate in the formation of transient multi-protein complexes with other enzymes in the various pathways diverging from the general phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of ...
biosynthetic pathway.
Localization
The enzyme is localized in thecytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
, associating with the endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ...
membrane. In another study, it was shown that CHS and CHI co-localize at the nucleus as well.
Active site
There are two distinct bi-lobedactive site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate ( binding site) ...
cavities located at the bottom edge of each monomer’s αβαβα core. Identical six-residue loops, which meet at the dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
interface, separate the two active sites from each other. The loops being with Thr132 in the active site and ends with a cis-peptide bond to Pro138. A Met137 residue plugs a hole in the other monomer’s active site. Therefore, the active site is buried except for a 16 Å CoA-binding tunnel that connects the catalytic surface to the outer surrounding milieu
The social environment, social context, sociocultural context or milieu refers to the immediate physical and social setting in which people live or in which something happens or develops. It includes the culture that the individual was educate ...
. The width of the tunnel is too narrow for the aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
substrates and products that must pass through it, implying that there must be some dynamic mobility within and around the tunnel when placed in solution.
The active site contains a conserved catalytic triad
A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, li ...
of Cys164, His303 and Asn336. These residues aid in multiple decarboxylation and condensation reactions, with Cys164 acting as the active site nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
. Phe215 and Phe265 are two other important amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
that act as “gatekeepers” to block the lower protein of the opening between the CoA-binding tunnel and the active site cavity. This limits the access of water to the active site while accommodating substrates and intermediates of varying shapes and sizes. Phe215 also orients the substrates at the active site during elongation of the polyketide intermediate.
Mechanism
The first step involves a transfer of a coumaroyl moiety from a 4-coumaroyl-CoA starter molecule to Cys164. Next, a series of condensation reactions of three acetate units from malonyl-CoA occurs, each proceeding through anacetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3 ...
derived from malonyl-CoA decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
. This extends the polyketide intermediate. After the generation of a thioester-linked tetraketide, a regiospecific C1,C6 Claisen condensation occurs, forming a new ring system to generate naringenin chalcone.
Regulation
Metabolic
CHS is noncompetitively inhibited by flavanoid pathway products such asnaringenin
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.
Structure
Naringenin has the skeleton structure of a flavanone with three hydro ...
and chalcone naringenin. Despite lack of direct evidence ''in vivo'', flavonoids are believed to accumulate in the cytosol to a level that blocks CHS activity to avoid toxic levels in plants.
Transcriptional
CHS is constitutively expressed in plants but can also be subject to induced expression through light/ UV light and well as in response to pathogens, elicitors and wounding. The ''CHS'' promoter contains a G-box motif with a sequence of CACGTG. This has been shown to play a role in response to light. Other light sensitive domains include Box I, Box II, Box III, Box IV or three copies of H-box (CCTACC). The chalcone synthasegene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
of '' Petunia'' plants is famous for being the first gene in which the phenomenon of RNA interference was observed; researchers intending to upregulate the production of pigments in light pink or violet flowers introduced a transgene
A transgene is a gene that has been transferred naturally, or by any of a number of genetic engineering techniques, from one organism to another. The introduction of a transgene, in a process known as transgenesis, has the potential to change t ...
for chalcone synthase, expecting that both the native gene and the transgene would express the enzyme and result in a more deeply colored flower phenotype
In genetics, the phenotype () is the set of observable characteristics or traits of an organism. The term covers the organism's morphology (biology), morphology or physical form and structure, its Developmental biology, developmental proc ...
. Instead the transgenic plants had mottled white flowers, indicating that the introduction of the transgene had downregulated or silenced chalcone synthase expression. Further investigation of the phenomenon indicated that the downregulation was due to post-transcriptional inhibition of the chalcone synthase gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. T ...
via an increased rate of messenger RNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the ...
degradation.
Disease relevance
CHS, as the first committed step in the flavonoid pathway, facilitate production of flavanoids, isoflavonoid-type phytoalexins and other metabolites to protect the plant from stress. CHS expression is also involved in the salicyclic acid defense pathway. Being aromatic compounds, flavonoids strongly absorb UV light through a photoreceptor-mediated mechanism which effectively protects the plants from DNA damage. CHS is involved in a broader, more general phenylpropanoid pathway which serve as precursors to a range of plant metabolites important to human health such as antioxidants, anti-inflammatory agents, antiallergens, and even antioncogenic products.Evolution
CHS belongs to a broader class of enzymes known as type III PKSs. Being the first enzyme of its type to be discovered, all other members are often labeled as “CHS-like.” Most or all of the divergent CHS-like enzymes characterized have arisen from extensive duplication and subsequent genetic variation of the ''chs'' gene. Duplication provides CHS activity with functional redundancy, allowing the ''chs'' gene to mutate without endangering flavonoid biosynthesis. These divergent enzymes differ from CHS in their preference for starter molecules, the number of acetyl additions (often through malonyl-CoA) and even in the mechanism of ring formation used to cyclize identical polyketide intermediates. The enzyme function of CHS and CHS-like enzymes function very similarly to fatty acid biosynthesis, but without the involvement of acyl-carrier proteins (ACP). Structural evidence suggests that these enzymes emerged by gain of function from ketoacyl synthase (KAS) III, an early stage enzyme of type IIfatty acid biosynthesis
Fatty is a derogatory term for someone who is obese. It may refer also to:
People
* Mai Fatty, Gambian politician
* Roscoe Arbuckle (1887–1933), American actor and comedian
* Fatty Briody (1858–1903), American Major League Baseball playe ...
.
Although higher plant chalcone synthases have been extensively studied, little information is available on the enzymes from bryophytes (primitive plants). Cloning of CHS from the moss ''Physcomitrella patens
''Physcomitrium patens'', (synonym: ''Physcomitrella patens'' ) the spreading earthmoss, is a moss (bryophyte) used as a model organism for studies on plant evolution, development, and physiology.
Distribution and ecology
''Physcomitrella p ...
'' revealed an important transition from the chalcone synthases present in microorganisms to those present in higher plants.
References
Literature
* *External links
BRENDA entry
{{Portal bar, Biology, border=no EC 2.3.1 Chalconoids metabolism Enzymes of known structure Dihydrochalcones metabolism