cytostructure on:
[Wikipedia]
[Google]
[Amazon]
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes.
In the 20th century, a variety of experimental techniques were developed to examine the 3D structures of biological molecules. The most prominent techniques are 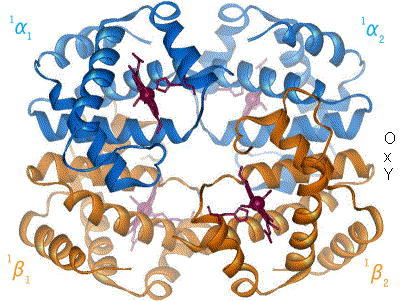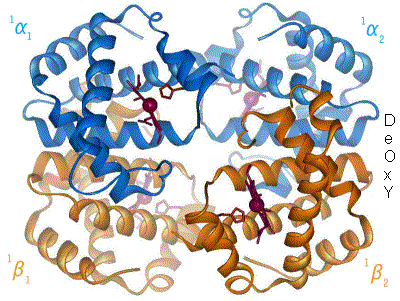
 Structural biologists have made significant contributions towards understanding the molecular components and mechanisms underlying human diseases. For example, cryo-EM and ssNMR have been used to study the aggregation of amyloid fibrils, which are associated with Alzheimer's disease,
Structural biologists have made significant contributions towards understanding the molecular components and mechanisms underlying human diseases. For example, cryo-EM and ssNMR have been used to study the aggregation of amyloid fibrils, which are associated with Alzheimer's disease,
''Nature: Structural & Molecular Biology'' magazine website
Journal of Structural Biology
Structural Biology - The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
''Structural Biology in Europe''
{{Authority control Molecular biology Protein structure Biophysics
X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
, nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
, and electron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a ...
. Through the discovery of X-rays
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
and its applications to protein crystals, structural biology was revolutionized, as now scientists could obtain the three-dimensional structures of biological molecules in atomic detail. Likewise, NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
allowed information about protein structure and dynamics to be obtained. Finally, in the 21st century, electron microscopy also saw a drastic revolution with the development of more coherent electron sources, aberration correction for electron microscopes, and reconstruction software that enabled the successful implementation of high resolution cryo-electron microscopy, thereby permitting the study of individual proteins and molecular complexes in three-dimensions at angstrom resolution.
With the development of these three techniques, the field of structural biology expanded and also became a branch of molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and phys ...
, biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology ...
, and biophysics
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. ...
concerned with the molecular structure of biological macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. ...
s (especially protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s, made up of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s, RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
or DNA, made up of nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecul ...
s, and membranes, made up of lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids incl ...
s), how they acquire the structures they have, and how alterations in their structures affect their function. This subject is of great interest to biologists because macromolecules carry out most of the functions of cells, and it is only by coiling into specific three-dimensional shapes that they are able to perform these functions. This architecture, the "tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
" of molecules, depends in a complicated way on each molecule's basic composition, or "primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
." At lower resolutions, tools such as FIB-SEM tomography have allowed for greater understanding of cells and their organelles in 3-dimensions, and how each hierarchical level of various extracellular matrices contributes to function (for example in bone). In the past few years it has also become possible to predict highly accurate physical molecular models to complement the experimental study of biological structures. Computational techniques such as Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of th ...
simulations can be used in conjunction with empirical structure determination strategies to extend and study protein structure, conformation and function.

History
In 1912Max Von Laue
Max Theodor Felix von Laue (; 9 October 1879 – 24 April 1960) was a German physicist who received the Nobel Prize in Physics in 1914 for his discovery of the diffraction of X-rays by crystals.
In addition to his scientific endeavors with con ...
directed X-Rays at crystallized copper sulfate generating a diffraction pattern. These experiments led to the development of X-Ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
, and its usage in exploring biological structures. In 1951, Rosalind Franklin and Maurice Wilkins used X-ray diffraction patterns to capture the first image of deoxyribonucleic acid (DNA). Francis Crick and James Watson modeled the double helical structure of DNA using this same technique in 1953 and received the Nobel Prize in Medicine along with Wilkins in 1962.
Pepsin crystals were the first proteins to be crystallized for use in X-Ray diffraction, by Theodore Svedberg who received the 1962 Nobel Prize in Chemistry. The first tertiary protein structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
, that of Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
, was published in 1958 by John Kendrew. During this time, modeling of protein structures was done using balsa wood or wire
Overhead power cabling. The conductor consists of seven strands of steel (centre, high tensile strength), surrounded by four outer layers of aluminium (high conductivity). Sample diameter 40 mm
A wire is a flexible strand of metal.
Wire is co ...
models. With the invention of modeling software such as CCP4 in the late 1970s, modeling is now done with computer assistance. Recent developments in the field have included the generation of X-ray free electron lasers, allowing analysis of the dynamics and motion of biological molecules, and the use of structural biology in assisting synthetic biology
Synthetic biology (SynBio) is a multidisciplinary area of research that seeks to create new biological parts, devices, and systems, or to redesign systems that are already found in nature.
It is a branch of science that encompasses a broad ran ...
.
In the late 1930s and early 1940s, the combination of work done by Isidor Rabi
Isidor Isaac Rabi (; born Israel Isaac Rabi, July 29, 1898 – January 11, 1988) was an American physicist who won the Nobel Prize in Physics in 1944 for his discovery of nuclear magnetic resonance, which is used in magnetic resonance i ...
, Felix Bloch, and Edward Mills Purcell led to the development of nuclear magnetic resonance (NMR). Currently, solid-state NMR is widely used in the field of structural biology to determine the structure and dynamic nature of proteins ( protein NMR).
In 1990, Richard Henderson produced the first three-dimensional, high resolution image of bacteriorhodopsin using cryogenic electron microscopy
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample so ...
(cryo-EM). Since then, cryo-EM has emerged as an increasingly popular technique to determine three-dimensional, high resolution structures of biological images.
More recently, computational methods have been developed to model and study biological structures. For example, molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of th ...
(MD) is commonly used to analyze the dynamic movements of biological molecules. In 1975, the first simulation of a biological folding process using MD was published in Nature. Recently, protein structure prediction was significantly improved by a new machine learning method called AlphaFold. Some claim that computational approaches are starting to lead the field of structural biology research.
Techniques
Biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include larg ...
s are too small to see in detail even with the most advanced light microscope
A microscope () is a laboratory instrument used to examine objects that are too small to be seen by the naked eye. Microscopy is the science of investigating small objects and structures using a microscope. Microscopic means being invisibl ...
s. The methods that structural biologists use to determine their structures generally involve measurements on vast numbers of identical molecules at the same time. These methods include:
* Mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
* Macromolecular crystallography
* Neutron diffraction
* Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
* Nuclear magnetic resonance spectroscopy of proteins (NMR)
* Electron paramagnetic resonance (EPR)
* Cryogenic Electron Microscopy (cryoEM)
* Electron crystallography and Microcrystal electron diffraction
* Multiangle light scattering
* Small angle scattering Small-angle scattering (SAS) is a scattering technique based on deflection of collimated radiation away from the straight trajectory after it interacts with structures that are much larger than the wavelength of the radiation. The deflection is sma ...
* Ultrafast laser spectroscopy
Ultrafast laser spectroscopy is a spectroscopic technique that uses ultrashort pulse lasers for the study of dynamics on extremely short time scales ( attoseconds to nanoseconds). Different methods are used to examine the dynamics of charge carrie ...
* Anisotropic terahertz microspectroscopy
* Two-dimensional infrared spectroscopy
* Dual-polarization interferometry and circular dichroism
Most often researchers use them to study the " native states" of macromolecules. But variations on these methods are also used to watch nascent or denatured molecules assume or reassume their native states. See protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reprodu ...
.
A third approach that structural biologists take to understanding structure is bioinformatics
Bioinformatics () is an interdisciplinary field that develops methods and software tools for understanding biological data, in particular when the data sets are large and complex. As an interdisciplinary field of science, bioinformatics combin ...
to look for patterns among the diverse sequence
In mathematics, a sequence is an enumerated collection of objects in which repetitions are allowed and order matters. Like a set, it contains members (also called ''elements'', or ''terms''). The number of elements (possibly infinite) is called ...
s that give rise to particular shapes. Researchers often can deduce aspects of the structure of integral membrane protein
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All ''transmembrane proteins'' are IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a sign ...
s based on the membrane topology predicted by hydrophobicity analysis
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with ...
. See protein structure prediction.
Applications
 Structural biologists have made significant contributions towards understanding the molecular components and mechanisms underlying human diseases. For example, cryo-EM and ssNMR have been used to study the aggregation of amyloid fibrils, which are associated with Alzheimer's disease,
Structural biologists have made significant contributions towards understanding the molecular components and mechanisms underlying human diseases. For example, cryo-EM and ssNMR have been used to study the aggregation of amyloid fibrils, which are associated with Alzheimer's disease, Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms becom ...
, and type II diabetes. In addition to amyloid proteins, scientists have used cryo-EM to produce high resolution models of tau filaments in the brain of Alzheimer's patients which may help develop better treatments in the future. Structural biology tools can also be used to explain interactions between pathogens and hosts. For example, structural biology tools have enabled virologists to understand how the HIV envelope
''Env'' is a viral gene that encodes the protein forming the viral envelope. The expression of the ''env'' gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
Analysis of the structure ...
allows the virus to evade human immune responses.
Structural biology is also an important component of drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.
Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by ...
. Scientists can identify targets using genomics, study those targets using structural biology, and develop drugs that are suited for those targets. Specifically, ligand- NMR, mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
, and X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
are commonly used techniques in the drug discovery process. For example, researchers have used structural biology to better understand Met, which is an important drug target for cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal bl ...
. Similar research has been conducted for HIV
The human immunodeficiency viruses (HIV) are two species of '' Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immu ...
targets to treat people with AIDS. Researchers are also developing new antimicrobials for mycobacterial infections using structure-driven drug discovery.
See also
*Primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
* Secondary structure
Protein secondary structure is the three dimensional form of ''local segments'' of proteins. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary struct ...
* Tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
* Quaternary structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also refe ...
* Structural domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of se ...
* Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have t ...
* Protein subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex.
Large assemblies of proteins such as viruses often use a small number of ...
* Molecular model
* Cooperativity
Cooperativity is a phenomenon displayed by systems involving identical or near-identical elements, which act dependently of each other, relative to a hypothetical standard non-interacting system in which the individual elements are acting indepen ...
* Chaperonin
* Structural genomics
* Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereo ...
* Resolution (electron density)
* Proteopedia The collaborative, 3D encyclopedia of proteins and other molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioc ...
.
* Protein structure prediction
References
External links
*''Nature: Structural & Molecular Biology'' magazine website
Journal of Structural Biology
Structural Biology - The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
''Structural Biology in Europe''
{{Authority control Molecular biology Protein structure Biophysics