Cyclopropane Carbon-carbon Bond Activation on:
[Wikipedia]
[Google]
[Amazon]
 In
In 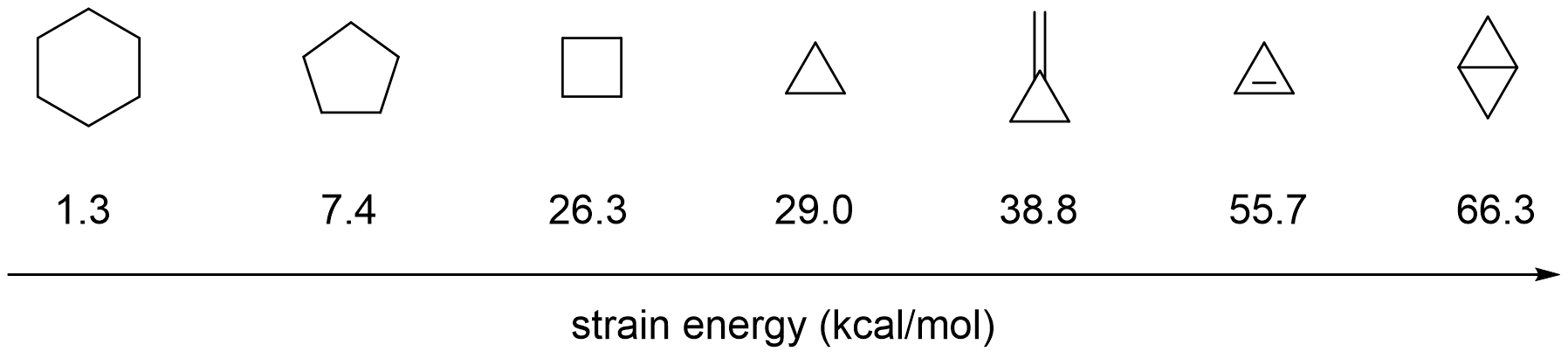 Two main approaches achieve C-C bond activation using a transition metal. One strategy is to increase the
Two main approaches achieve C-C bond activation using a transition metal. One strategy is to increase the

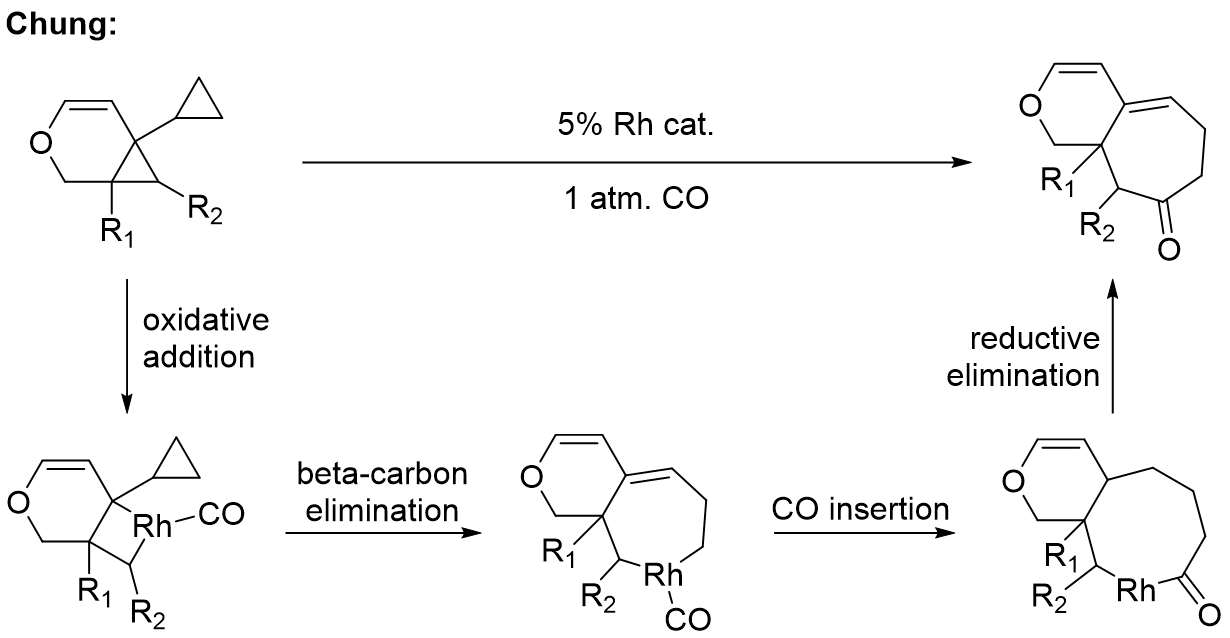
 Using the same rhodium(I) catalyst and C-C bond activation strategy one can access compounds with fused rings. Once again the reaction involves oxidative addition to give a rhodacyclobutane eventually affording a rhodacycloheptene intermediate. Insertion of carbon monoxide into one of the carbon-rhodium bonds form a rhodacyclooctenone intermediate that can reductively eliminate to yield a 6,7-fused ring system. The authors propose that the regioselectivity of the initial oxidative addition is controlled by coordination of the endocyclic double bond to the rhodium catalyst.
Using the same rhodium(I) catalyst and C-C bond activation strategy one can access compounds with fused rings. Once again the reaction involves oxidative addition to give a rhodacyclobutane eventually affording a rhodacycloheptene intermediate. Insertion of carbon monoxide into one of the carbon-rhodium bonds form a rhodacyclooctenone intermediate that can reductively eliminate to yield a 6,7-fused ring system. The authors propose that the regioselectivity of the initial oxidative addition is controlled by coordination of the endocyclic double bond to the rhodium catalyst.
 With the metallacyclobutane intermediate, 1,2-migratory insertion into an
With the metallacyclobutane intermediate, 1,2-migratory insertion into an 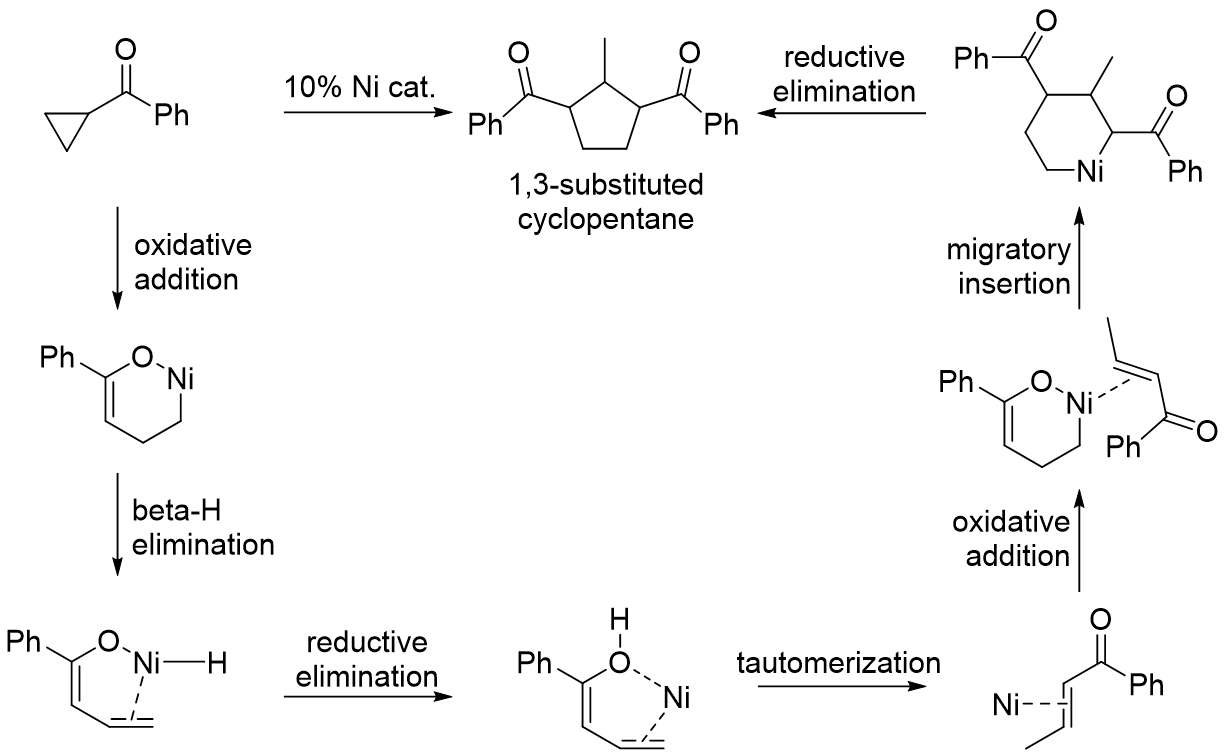






 In
In organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength
Strength may refer to:
Physical strength
*Physical strength, as in people or animals
*Hysterical strength, extreme strength occurring when people are in life-and-death situations
*Superhuman strength, great physical strength far above human ca ...
of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.
 Two main approaches achieve C-C bond activation using a transition metal. One strategy is to increase the
Two main approaches achieve C-C bond activation using a transition metal. One strategy is to increase the ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are su ...
and the other is to stabilize the resulting cleaved C-C bond complex (e.g. through aromatization or chelation). Because of the large ring strain energy of cyclopropanes (29.0 kcal per mole), they are often used as substrates for C-C activation through oxidative addition of a transition metal into one of the three C-C bonds leading to a metallacyclobutane intermediate.
Substituents on the cyclopropane affect the course of its activation.
Reaction scope
Cyclopropane
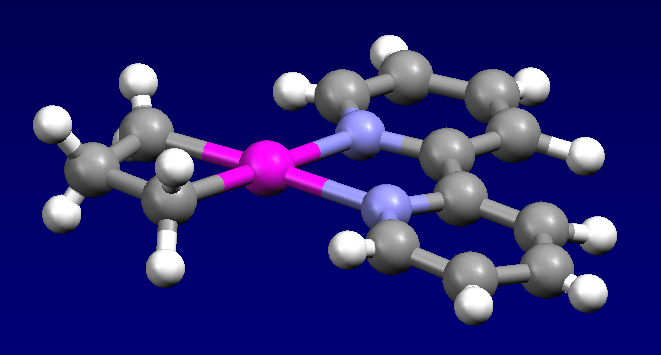
The first example of cyclopropane being activated by a metal complex was reported in 1955, involving the reaction of cyclopropane and hexachloroplatinic acid. This reaction produces the polymeric platinacyclobutane complex Pt(C3H6)Cl2. The bis(pyridine) adduct of this complex was characterized by X-ray crystallography. The electrophile Cp*Ir(PMe3)(Me)OTf reacts with cyclopropane to give the allyl complex: :Cp*Ir(PMe3)(Me)OTf + C3H6 → p*Ir(PMe3)(η3-C3H5)Tf + CH4
Fused and spiro-cyclopropanes
Rhodium-catalyzed C-C bondactivation of strainedspiropentane
Spiropentane is a hydrocarbon with formula . It is the simplest Spiro compound, spiro-connected cycloalkane, a triangulane.
It took several years after the discovery in 1887 until the structure of the molecule was determined. According to the nome ...
s leads to a cyclopentenone
2-Cyclopentenone is a ketone with chemical formula and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone contains two functional groups, a k ...
s. In terms of mechanism, the reaction proceeds by apparent oxidative addition of the 4-5 carbon-carbon bond, leading to a rhodacyclobutane intermediate. In the presence of carbon monoxide, migratory insertion of CO into one of the carbon-rhodium bonds gives a rhodacyclopentanone intermediate. Beta-carbon elimination to form an alkene from the other carbon-rhodium bond leads to a rhodacyclohexanone intermediate with an exocyclic double bond. Reductive elimination of the two carbon-rhodium bonds followed by isomerization of the exocyclic double bond leads to the desired beta-substituted cyclopentenone
2-Cyclopentenone is a ketone with chemical formula and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone contains two functional groups, a k ...
product. This reaction was applied to the total synthesis of (±)-β-cuparenone.
 Using the same rhodium(I) catalyst and C-C bond activation strategy one can access compounds with fused rings. Once again the reaction involves oxidative addition to give a rhodacyclobutane eventually affording a rhodacycloheptene intermediate. Insertion of carbon monoxide into one of the carbon-rhodium bonds form a rhodacyclooctenone intermediate that can reductively eliminate to yield a 6,7-fused ring system. The authors propose that the regioselectivity of the initial oxidative addition is controlled by coordination of the endocyclic double bond to the rhodium catalyst.
Using the same rhodium(I) catalyst and C-C bond activation strategy one can access compounds with fused rings. Once again the reaction involves oxidative addition to give a rhodacyclobutane eventually affording a rhodacycloheptene intermediate. Insertion of carbon monoxide into one of the carbon-rhodium bonds form a rhodacyclooctenone intermediate that can reductively eliminate to yield a 6,7-fused ring system. The authors propose that the regioselectivity of the initial oxidative addition is controlled by coordination of the endocyclic double bond to the rhodium catalyst.

Cyclopropyl halides
Nickel(0) complexes oxidatively cleave halocyclopropanes to give allyl)Ni(II) halides.Cyclopropylketones
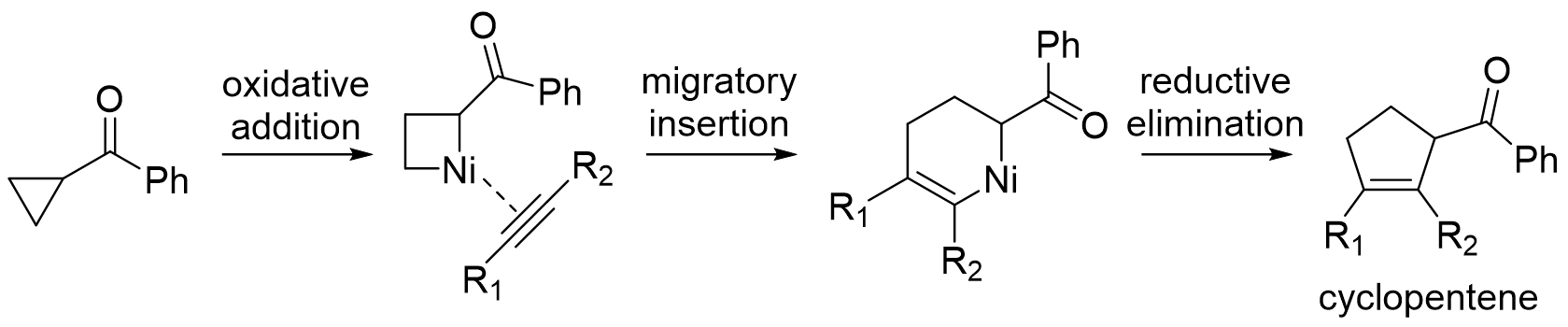
With cyclopropylketones, transition metal can coordinate to the ketone to direct oxidative addition into the proximal C-C bond. The resulting metallacyclobutane intermediate can be in equilibrium with the six-membered alkyl metal enolate depending on presence of aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
(e.g. dimethylaluminum chloride).
 With the metallacyclobutane intermediate, 1,2-migratory insertion into an
With the metallacyclobutane intermediate, 1,2-migratory insertion into an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
followed by reductive elimination yields a substituted cyclopentene product. Examples of intramolecular reactions with a tethered alkyne and intermolecular reactions with a nontethered alkyne both exist with use of a nickel or rhodium catalyst. With the six-membered alkyl metal enolate intermediate, dimerization
A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im ...
or reaction with an added alpha-beta unsaturated ketone yields a 1,3-substituted cyclopentane product.


Cyclopropylimines
Oxidative addition into cyclopropylimines gives a metalloenamine intermediate similar to oxidative addition to cyclopropylketones giving alkylmetalloenolates. These intermediates can also reaction with alpha-beta unsaturated ketones to give disubstituted cyclopentane products following reductive elimination. With rhodium, the intermediate metalloenamine reacts with tethered alkynes. and alkenes to give cyclized products such aspyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
s and cyclohexenone
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances. It is colorless liquid, but commercial samples are often yellow.
Industrially, ...
s, respectively.

Alylidenecyclopropanes
Alkylidenecyclopropanes more readily undergo C-C bond oxidative addition than cyclopropanes. Following oxidative addition, 1,2-insertion mechanisms are common and reductive elimination yields the desired product. The 1,2-insertion step usually occurs with an alkyne, alkene, or allene and the final product is often a 5 or 7 membered ring. Six-membered rings may be formed after dimerization of the metallocyclobutane intermediate with another alkylidenecyclopropane substrate and subsequent reductive elimination. Common transition metals utilized with alkylidenecyclopropanes are nickel, rhodium, and palladium. It has been shown that the metallacyclobutane intermediate following oxidative addition to the distal C-C bond can isomerize.


Vinylcyclopropanes
Oxidative addition of vinylcyclopropanes primarily occurs at the proximal position, giving pi-allyl intermediates. Through subsequent insertion reactions (e.g. withalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, alkenes, and carbon monoxide), rings of various sizes and fused ring systems can be formed.

Cyclopropenes
Oxidative addition into cyclopropenes normally occurs at the less hindered position to yield the metallacyclobutane. This reaction can result in formation of cyclopentadienones, cyclohexenones, and phenols.
References
{{Reflist, 30em Chemical bond properties Cyclopropanes Organometallic chemistry