Cuprospinel on:
[Wikipedia]
[Google]
[Amazon]
Cuprospinel is a
 Another example for MCR utilizing CuFe2O4 was published in a research towards the A3 coupling of
Another example for MCR utilizing CuFe2O4 was published in a research towards the A3 coupling of 


 The role of copper has been further emphasized in the coupling reaction of ortho-arylated phenols and dialkylformamides. It was observed that there was a single-electron oxidative addition of copperII to copperIII through a radical step, then transformed back to copperI by reductive elimination in the presence of either oxygen or peroxide. Catalyst can be reused 9 times without significant loss in catalytic activities.
The role of copper has been further emphasized in the coupling reaction of ortho-arylated phenols and dialkylformamides. It was observed that there was a single-electron oxidative addition of copperII to copperIII through a radical step, then transformed back to copperI by reductive elimination in the presence of either oxygen or peroxide. Catalyst can be reused 9 times without significant loss in catalytic activities.

mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ( ...
. Cuprospinel is an inverse spinel
Spinel () is the magnesium/aluminium member of the larger spinel group of minerals. It has the formula in the cubic crystal system. Its name comes from the Latin word , which means ''spine'' in reference to its pointed crystals.
Properties
S ...
with the chemical formula CuFe2O4, where copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
substitutes some of the iron cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s in the structure.
Its structure is similar to that of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the ...
, Fe3O4, yet with slightly different chemical and physical properties due to the presence of copper.
The type locality of cuprospinel is Baie Verte, Newfoundland
Newfoundland and Labrador (; french: Terre-Neuve-et-Labrador; frequently abbreviated as NL) is the easternmost province of Canada, in the country's Atlantic region. The province comprises the island of Newfoundland and the continental region ...
, Canada, where the mineral was found in an exposed ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
dump. The mineral was first characterized by Ernest Henry Nickel
Ernest (Ernie) Henry Nickel (born Ernst Heinrich Nickel on 31 August 1925 in Louth, Ontario, died on 18 July 2009) was a mineralogist from Canada who emigrated to Australia. He is best known as an editor of the ninth edition of the Nickel-Strun ...
, a mineralogist
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the proces ...
with the Department of Energy, Mines and Resources in Australia, in 1973. Cuprospinel is also found in other places, for example, in Hubei province
Hubei (; ; alternately Hupeh) is a landlocked province of the People's Republic of China, and is part of the Central China region. The name of the province means "north of the lake", referring to its position north of Dongting Lake. The prov ...
, China and at Tolbachik
Tolbachik (russian: Толбачик) is a complex volcano, volcanic complex on the Kamchatka Peninsula in the far east of Russia. It consists of two volcanoes, Plosky (''flat'') Tolbachik (3,085 m) and Ostry (''sharp'') Tolbachik (3,682 m), whic ...
volcano in Kamchatka
The Kamchatka Peninsula (russian: полуостров Камчатка, Poluostrov Kamchatka, ) is a peninsula in the Russian Far East, with an area of about . The Pacific Ocean and the Sea of Okhotsk make up the peninsula's eastern and wes ...
, Russia.
Structural properties
Cuprospinel, like many other spinels has the general formula AB2O4. Yet, cuprospinel is an ''inverse'' spinel in that its ''A'' element, in this case copper (Cu2+), only occupies octahedral sites in the structure and the ''B'' element,iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
(Fe2+ and Fe3+), is split between the octahedral and tetrahedral sites in the structure. The Fe2+ species will occupy some of the octahedral sites and there will only be Fe3+ at the tetrahedral sites.
Cuprospinel adopts both cubic and tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square ...
phases at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, yet as temperature is elevated the cubic form is most stable.
Magnetic properties
nanoparticles have been characterized as a superparamagnetic material with saturated magnetization of ,remnant magnetization
Remnant or remnants may refer to:
Religion
* Remnant (Bible), a recurring theme in the Bible
* Remnant (Seventh-day Adventist belief), the remnant theme in the Seventh-day Adventist Church
* ''The Remnant'' (newspaper), a traditional Catholic ne ...
and coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in ...
. The magnetic properties of are correlated with the size of particles. Particularly, the decreasing in saturated magnetization and remanence correspond to the decreasing in the size of particles, whereas the coercivity increases.
Solid phase synthesis
Spinel CuFe2O4 can be synthesized by solid phase synthesis at high temperature. In a particular procedure for this type of synthesis, thestoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
mixture of Cu(CH3COO)2· and FeC2O2 is ground together and stirred in a solvent. After evaporation of the solvent, the resulting powder is heated in a furnace at constant temperature around 900 °C in normal air-atmosphere environment. Then the resulting product is slowly cooled to room temperature in order to obtain the desired stable spinel structure.
Hydrothermal treatment of a precipitate in TEG
A method combining a first precipitation step at room temperature intriethylene glycol
Triethylene glycol, TEG, or triglycol is a colorless odorless viscous liquid with molecular formula HOCH2CH2OCH2CH2OCH2CH2OH. It is used as a plasticizer for vinyl polymers. It is also used in air sanitizer products, such as "Oust" or "Clean and ...
(TEG), a viscous
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inter ...
and highly hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
liquid with an elevated , followed by a thermal treatment at elevated temperature is an effective way to synthesize spinel oxide, especially copper iron oxide. Typically, NaOH is first added dropwise to a solution of Fe3+ (Fe(NO3)3 or Fe(acac)3) and Cu2+ (Cu(NO3)2 or CuCl2) in triethylene glycol
Triethylene glycol, TEG, or triglycol is a colorless odorless viscous liquid with molecular formula HOCH2CH2OCH2CH2OCH2CH2OH. It is used as a plasticizer for vinyl polymers. It is also used in air sanitizer products, such as "Oust" or "Clean and ...
at room temperature with constant stirring until a reddish-black precipitate completely form. The resulting viscous suspension is then placed in an ultrasonic bath to be properly dispersed, . The final product is then washed in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
, ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
, ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
and deionized water
Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently puri ...
, and then dried under vacuum to obtain oxide particles.
Uses
Cuprospinel is used in various industrial processes as acatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. An example is the water–gas shift reaction
The water-gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen:
: CO + H2O CO2 + H2
The water gas shift reaction was discovered by Italian physicist Felice Fontana in 1780. ...
:
: H2O''(v)'' + CO''(g)'' → CO2''(g)'' + H2''(g)''
This reaction is particularly important for hydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of h ...
and enrichment.
The interest of cuprospinel arises in that magnetite is a widely used catalyst for many industrial chemical reactions, such as the Fischer–Tropsch process
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperat ...
, the Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
and the water-gas shift reaction. It has been shown that doping magnetite with other elements gives it different chemical and physical properties; these different properties sometimes allow the catalyst to work more efficiently. As such, cuprospinel is essentially magnetite doped with copper and this enhances magnetite's water gas shift properties as a heterogeneous catalyst.
Recyclable catalyst for organic reactions
Recent years, various research towards the heterogeneous catalytic ability of CuFe2O4 in organic synthesis have been published ranging from traditional reactions to modern organometallic transformation. By taking advantages of magnetic nature, the catalyst can be separated simply by external magnetism, which can overcome the difficulty to separate nano-scaled metal catalyst from the reaction mixture. Particularly, only by applying magnetic bar at the outer vessel, the catalyst can easily be held at the edge of container while removing solution and washing particles. The obtained particles can be readily used for the next catalyst cycles. Moreover, the catalytic site can be exploited in either cooper or iron center because of the large-surface area of nanoparticles, leading to wide scope to apply this material in various types of reactions.Catalyst for multi-component reaction (MCR)
Nano CuFe2O4 can be utilized as acatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
in a ''one-pot'' synthesis of fluorine containing spirohexahydropyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
derivatives. It has also been observed that the catalyst can be reused five times without significant loss in catalytic activity after each runs. In the reaction, iron plays a vital role in the coordination with the carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
in order to increase the electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
property, which can facilitate the reaction conditions and increase the reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
.
 Another example for MCR utilizing CuFe2O4 was published in a research towards the A3 coupling of
Another example for MCR utilizing CuFe2O4 was published in a research towards the A3 coupling of aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
with phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
I ...
to give the corresponding propargylamines. The catalyst can be reused three times without remarkable reduce in reaction yield
In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage. Yield is one of the pr ...
.

Catalyst for C-O cross coupling
Pallapothula and coworkers demonstrated CuFe2O4 is an efficient catalyst for C-O cross-coupling between phenols and aryl halides. The catalyst exhibited superior activity in comparison with other nanoparticles oxides such as Co3O4, SnO2, Sb2O3. Moreover, the catalyst can benefit in applying C-O cross-coupling on alkyl alcohols, leading to widening scope for the transformation. :
Catalyst for C-H activation
Nano CuFe2O4 catalyst was demonstrated its activity for C-H activation in Mannich type reaction. In the mechanistic study, the copper play a significant role in both generate radical from TBHP and activate C-H from substituted alkyne. In this reaction, iron center was considered as a magnetic source and this hypothesis was proved by the experiment, in which magnetic Fe3O4 had been used but failed to catalyze reaction in the absence of copper center. :
Other reactions
CuFe2O4 can also be applied for C-C cleavage α-arylation between acetylacetone with iodobenzene. The phenylacetone product was obtained with excellent yield at 99% and 95% selectivity observed for principal product compared to 3-phenyl-2,4-pentanedione as the byproduct. The XRD results were observed that crystal structure of catalyst remained unchanged after the sixth run while catalytic activity slightly decreases at 97% conversion in the final run. In this reaction, the mechanistic study showed the catalytic cycle started from CuII to CuI and then oxidized to CuII by aryl iodine. The role of copper has been further emphasized in the coupling reaction of ortho-arylated phenols and dialkylformamides. It was observed that there was a single-electron oxidative addition of copperII to copperIII through a radical step, then transformed back to copperI by reductive elimination in the presence of either oxygen or peroxide. Catalyst can be reused 9 times without significant loss in catalytic activities.
The role of copper has been further emphasized in the coupling reaction of ortho-arylated phenols and dialkylformamides. It was observed that there was a single-electron oxidative addition of copperII to copperIII through a radical step, then transformed back to copperI by reductive elimination in the presence of either oxygen or peroxide. Catalyst can be reused 9 times without significant loss in catalytic activities.

Synergistic effect of catalytic activity
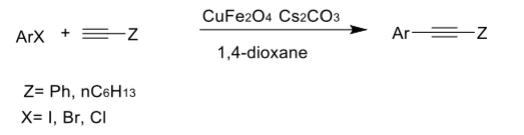
Notably, synergistic effect was demonstrated for the case of CuFe2O4 in Sonogashira reaction. Both Fe and Cu center contribute to catalytic activity of the transformation between aryl halide and substituted alkynes. The product was obtained with 70% yield in the presence of Nano CuFe2O4, while only 25% yield and <1% yield observed when using CuO and Fe3O4 respectively.
Mechanism of action of the catalyst
As can be noted in the examples shown above, many molecules involved in the reactions catalyzed by CuFe2O4 have a carbonyl group (C=O) or amine group (-NH2), which have electron lone pairs. These lone pairs are used to be adsorbed at the surface of the empty 3d orbital in the catalyst, and thus activate the molecules for the intended reactions. Other molecules containing functional groups with electron lone pairs such as nitro (NO2) and thiol (RS-H) also are activated by the catalyst. Species forming containing a single unpaired electron such as TEMPO or peroxymonosulphate are also adsorbed and activated to promote some organic reactions.References
{{Reflist, refs= {{cite journal , last1=Tamaddon , first1=Fatemeh , last2=Amirpoor , first2=Farideh , year=2013 , title=Improved Catalyst-Free Synthesis of Pyrrole Derivatives in Aqueous Media , journal=Synlett
''Synlett'' is an international scientific journal for accounts and rapid communications of original contributions of fundamental research in synthetic organic chemistry. The impact factor of this journal is 2.419 (2017). ''Nature'' featured a br ...
, volume=24 , issue=14 , pages=1791–1794
, doi=10.1055/s-0033-1339294
Catalysis
Spinel group
Iron(III) compounds