Crossover Junction Endodeoxyribonuclease on:
[Wikipedia]
[Google]
[Amazon]
Crossover junction endodeoxyribonuclease, also known as Holliday junction resolvase, Holliday junction endonuclease, Holliday junction-cleaving endonuclease, Holliday junction-resolving endoribonuclease, crossover junction endoribonuclease, and cruciform-cutting endonuclease, is an enzyme involved in DNA repair and homologous recombination. Specifically, it performs endonucleolytic cleavage that results in single-stranded crossover between two homologous DNA molecules at the
 Crossover junction endodeoxyribonucleases also play key roles in DNA repair. During
Crossover junction endodeoxyribonucleases also play key roles in DNA repair. During

Holliday junction
A Holliday junction is a branched nucleic acid structure that contains four double-stranded arms joined. These arms may adopt one of several conformations depending on buffer salt concentrations and the sequence of nucleobases closest to the ju ...
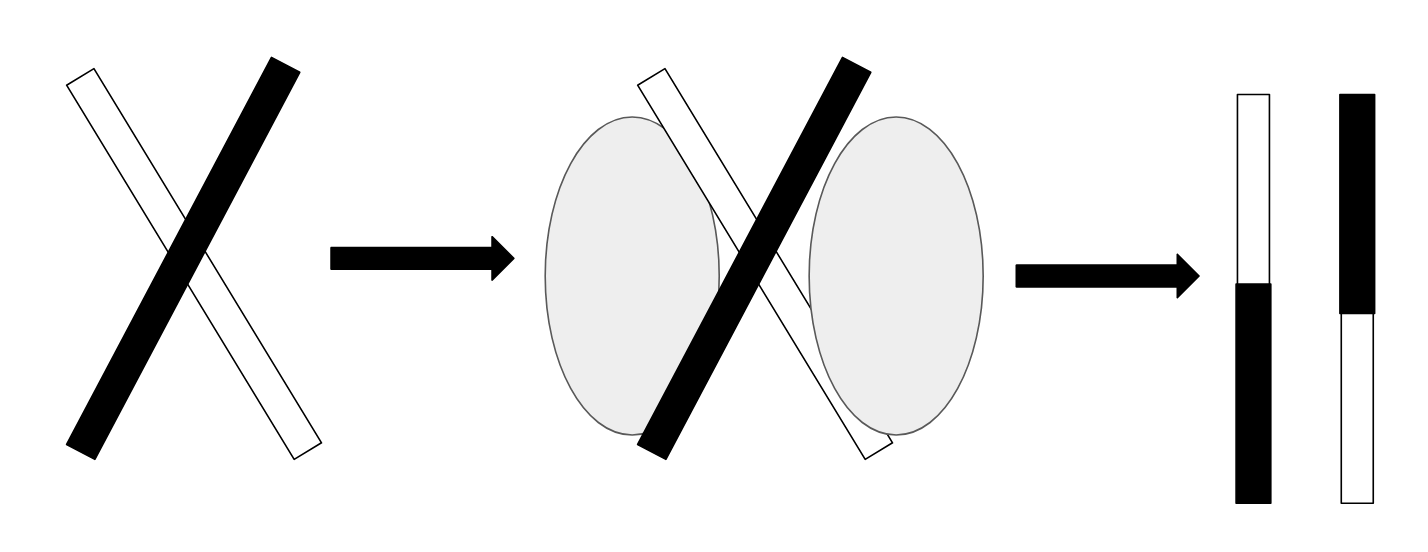
to produce recombinant DNA products for chromosomal segregation. This process is known as Holliday junction resolution.
Biological Function
TheHolliday junction
A Holliday junction is a branched nucleic acid structure that contains four double-stranded arms joined. These arms may adopt one of several conformations depending on buffer salt concentrations and the sequence of nucleobases closest to the ju ...
is a structure that forms during genetic recombination
Genetic recombination (also known as genetic reshuffling) is the exchange of genetic material between different organisms which leads to production of offspring with combinations of traits that differ from those found in either parent. In eukaryo ...
, and links two double-stranded DNA molecules with a single-stranded crossover, which form during mitotic and meiotic
Meiosis (; , since it is a reductional division) is a special type of cell division of germ cells in sexually-reproducing organisms that produces the gametes, such as sperm or egg cells. It involves two rounds of division that ultimately res ...
recombination. Crossover junction endodeoxyribonucleases catalyze Holiday junction resolution, which is the formation of separate recombinant DNA molecules and chromosomal separation after the crossover event at the Holliday junction. Crossover junction endodeoxyribonucleases with Holliday Junction resolution function have been identified in all three domains of life - bacteria, archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
, and eukarya. RuvC in bacteria, CCE1 in Saccharomyces cerevisiae, and GEN1 in humans are all crossover junction endodeoxyribonucleases that perform Holliday Junction resolution. Holliday junction resolution catalyzed by crossover junction endodeoxyribonuclease is shown in the figure below.
 Crossover junction endodeoxyribonucleases also play key roles in DNA repair. During
Crossover junction endodeoxyribonucleases also play key roles in DNA repair. During cell growth
Cell growth refers to an increase in the total mass of a cell, including both cytoplasmic, nuclear and organelle volume. Cell growth occurs when the overall rate of cellular biosynthesis (production of biomolecules or anabolism) is greater than ...
and meiosis, DNA double-strand breaks (DSBs) often occur, and are usually repaired by homologous recombination. Because Crossover junction endodeoxyribonucleases perform Holliday Junction resolution, a crucial step of homologous recombination, they are therefore involved in repair of DSBs.
Structure
E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
RuvC, a Crossover junction endodeoxyribonuclease, is a small protein of about 20 kD, and its active form is a dimer that requires and binds a magnesium ion RuvC is a 3-layer alpha-beta sandwich with a beta-sheet between 5 alpha-helices
. The enzyme contains two binding channels that contact the backbones of the Holliday junction over seven nucleotides. A Holliday junction resolvase enzyme has also been identified in archaea in Pyrococcus furiosus cells - it is encoded by a gene called hjc and is composed of 123 amino acids
.
A figure of Thermus thermophilus
''Thermus thermophilus'' is a Gram-negative bacterium used in a range of biotechnological applications, including as a model organism for genetic manipulation, structural genomics, and systems biology. The bacterium is extremely thermophilic ...
RuvC in complex with a Holliday junction is shown below.

Mechanism
These enzymes are highly selective for branched DNA, although induced fit occurs in the enzyme-substrate (resolvase-Holloday Junction) complex formation. Much remains unknown about the exact mechanism of action, but it is known that bacteria,bacteriophages
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacterio ...
and archaea catalyze Holliday junction resolution by introducing symmetric nicks across the Holliday junction
. Analysis of crossover junction endodeoxyribonucleases from bacteriophages (T7 endonuclease I), bacteria (RuvC), fungi (GEN1) and humans (hMus81-Eme1) have revealed that the enzymes function in dimers, and part of the resolution reaction takes place in a partially dissociated enzyme-substrate intermediate.
Human Relevance
After a 20-year search, in 2008, a human crossover junction endodeoxyribonuclease, GEN1, was finally identified . GEN1 performs similar functions and operates by similar mechanisms as previously studied Crossover junction endodeoxyribonuclease in bacteria, archaea, and other eukarya. The enzyme is thought to play a role in Bloom's syndrome. It has been proposed that Bloom's syndrome involves the induction of DSBs via an unidentified Holliday junction resolvase. It has also been shown that overexpression of Holliday Junction resolvase function is correlated with RAD51-overexpressing cancers.References
External links
* {{Portal bar, Biology, border=no EC 3.1.22