Crabbé Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Crabbé reaction (or Crabbé allene synthesis, Crabbé–Ma allene synthesis) is an
 Despite the excellent result for the substrate shown, yields were highly dependent on substrate structure and the scope of the process was narrow. The author noted that iron salts were completely ineffective, while cupric and cuprous chloride and bromide, as well as silver nitrate provided the desired product, but in lower yield under the standard conditions.
Despite the excellent result for the substrate shown, yields were highly dependent on substrate structure and the scope of the process was narrow. The author noted that iron salts were completely ineffective, while cupric and cuprous chloride and bromide, as well as silver nitrate provided the desired product, but in lower yield under the standard conditions.
 Shengming Ma (麻生明) and coworkers at the Shanghai Institute of Organic Chemistry (SIOC,
Shengming Ma (麻生明) and coworkers at the Shanghai Institute of Organic Chemistry (SIOC, 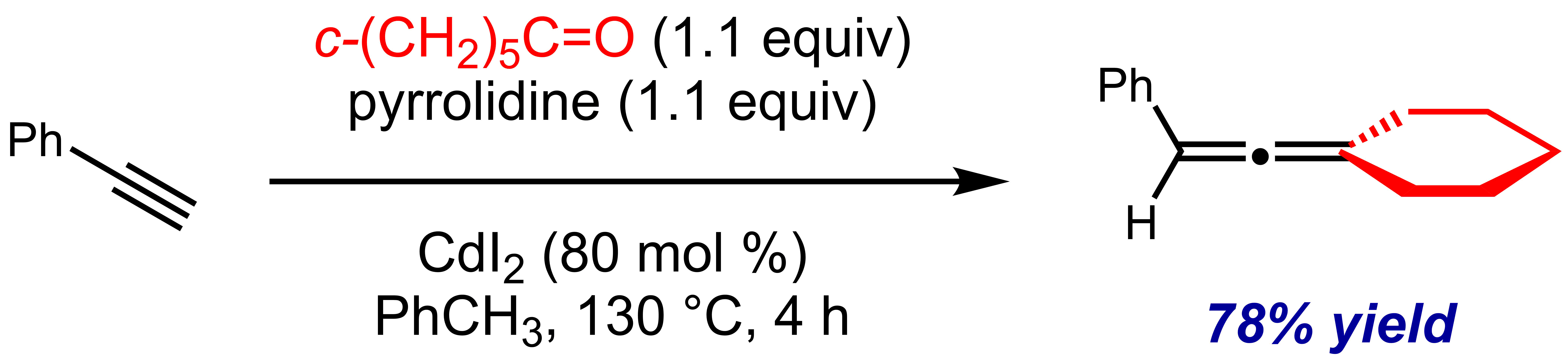 The Crabbé reaction is applicable to a limited range of ketone substrates for the synthesis of trisubstituted allenes; however, a near stoichiometric quantity (0.8 equiv) of cadmium iodide (CdI2) is needed to promote the reaction. Alternatively, the use of cuprous bromide and zinc iodide sequentially as catalysts is also effective, provided the copper catalyst is filtered before zinc iodide is added.
The Crabbé reaction is applicable to a limited range of ketone substrates for the synthesis of trisubstituted allenes; however, a near stoichiometric quantity (0.8 equiv) of cadmium iodide (CdI2) is needed to promote the reaction. Alternatively, the use of cuprous bromide and zinc iodide sequentially as catalysts is also effective, provided the copper catalyst is filtered before zinc iodide is added.
 (The copper catalyst is shown simply as "CuBr" or "Cu+", omitting any additional amine or halide ligands or the possibility of dinuclear interactions with other copper atoms. Condensation of formaldehyde and diisopropylamine to form the iminium ion and steps involving complexation and decomplexation of Cu+ are also omitted here for brevity.)
Since 2012, Ma has reported several catalytic enantioselective versions of the Crabbé reaction in which chiral PINAP (aza-BINAP) based ligands for copper are employed. The stepwise application of copper and zinc catalysis was required: the copper promotes the Mannich-type condensation, while subsequent one-step addition of zinc iodide catalyzes the imino-retro-ene reaction.
(The copper catalyst is shown simply as "CuBr" or "Cu+", omitting any additional amine or halide ligands or the possibility of dinuclear interactions with other copper atoms. Condensation of formaldehyde and diisopropylamine to form the iminium ion and steps involving complexation and decomplexation of Cu+ are also omitted here for brevity.)
Since 2012, Ma has reported several catalytic enantioselective versions of the Crabbé reaction in which chiral PINAP (aza-BINAP) based ligands for copper are employed. The stepwise application of copper and zinc catalysis was required: the copper promotes the Mannich-type condensation, while subsequent one-step addition of zinc iodide catalyzes the imino-retro-ene reaction.
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
that converts a terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
and aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
(or, sometimes, a ketone) into an allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as diene#Classes, cumulated dienes. The parent compound of this class is propa ...
in the presence of a soft Lewis acid catalyst (or stoichiometric promoter) and secondary amine. Given continued developments in scope and generality, it is a convenient and increasingly important method for the preparation of allenes, a class of compounds often viewed as exotic and synthetically challenging to access.
Overview and scope
The transformation was discovered in 1979 by Pierre Crabbé and coworkers at the Université Scientifique et Médicale (currently merged intoUniversité Grenoble Alpes
The Université Grenoble Alpes (UGA, French: meaning "''Grenoble Alps University''") is a public research university in Grenoble, France. Founded in 1339, it is the third largest university in France with about 60,000 students and over 3,000 resea ...
) in Grenoble, France. As initially discovered, the reaction was a one-carbon homologation reaction (the Crabbé homologation) of a terminal alkyne into a terminal allene using formaldehyde as the carbon source, with diisopropylamine as base and copper(I) bromide as catalyst.
 Despite the excellent result for the substrate shown, yields were highly dependent on substrate structure and the scope of the process was narrow. The author noted that iron salts were completely ineffective, while cupric and cuprous chloride and bromide, as well as silver nitrate provided the desired product, but in lower yield under the standard conditions.
Despite the excellent result for the substrate shown, yields were highly dependent on substrate structure and the scope of the process was narrow. The author noted that iron salts were completely ineffective, while cupric and cuprous chloride and bromide, as well as silver nitrate provided the desired product, but in lower yield under the standard conditions.
 Shengming Ma (麻生明) and coworkers at the Shanghai Institute of Organic Chemistry (SIOC,
Shengming Ma (麻生明) and coworkers at the Shanghai Institute of Organic Chemistry (SIOC, Chinese Academy of Sciences
The Chinese Academy of Sciences (CAS); ), known by Academia Sinica in English until the 1980s, is the national academy of the People's Republic of China for natural sciences. It has historical origins in the Academia Sinica during the Republ ...
) investigated the reaction in detail, including clarifying the critical role of the base, and developed conditions that exhibited superior functional-group compatibility and generally resulted in higher yields of the allene. One of the key changes was the use of dicyclohexylamine
Dicyclohexylamine is a secondary amine with the chemical formula HN(C6H11)2. It is a colorless liquid, although commercial samples can appear yellow. It has a fishy odor, typical for amines. It is sparingly soluble in water. As an amine, it is an ...
as the base. In another important advance, the Ma group found that the combination of zinc iodide and morpholine allowed aldehydes besides formaldehyde, including benzaldehyde derivatives and a more limited range of aliphatic aldehydes, to be used as coupling partners, furnishing 1,3-disubstituted allenes via an alkyne-aldehyde coupling method of substantial generality and utility. A separate protocol utilizing copper catalysis and a fine-tuned amine base was later developed to obtain better yields for aliphatic aldehydes.
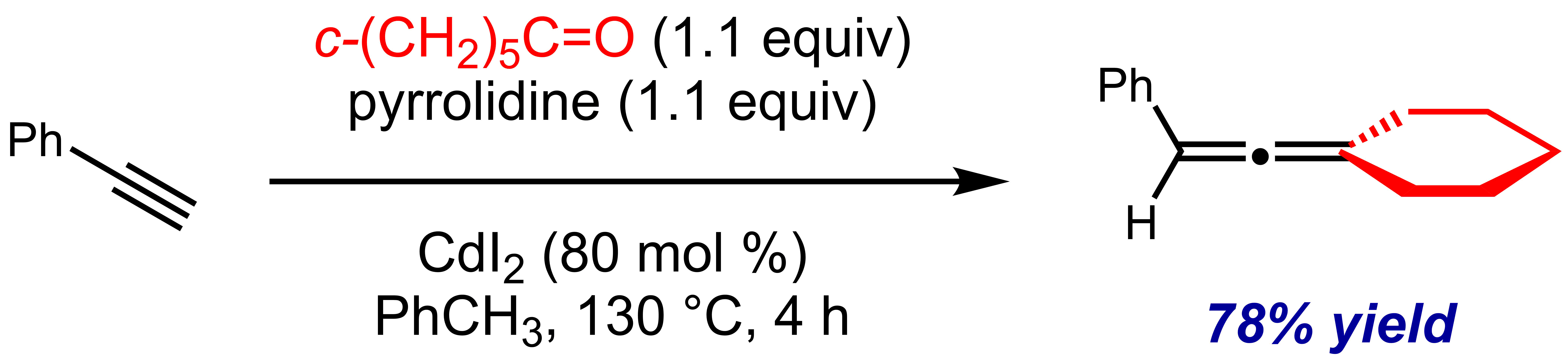 The Crabbé reaction is applicable to a limited range of ketone substrates for the synthesis of trisubstituted allenes; however, a near stoichiometric quantity (0.8 equiv) of cadmium iodide (CdI2) is needed to promote the reaction. Alternatively, the use of cuprous bromide and zinc iodide sequentially as catalysts is also effective, provided the copper catalyst is filtered before zinc iodide is added.
The Crabbé reaction is applicable to a limited range of ketone substrates for the synthesis of trisubstituted allenes; however, a near stoichiometric quantity (0.8 equiv) of cadmium iodide (CdI2) is needed to promote the reaction. Alternatively, the use of cuprous bromide and zinc iodide sequentially as catalysts is also effective, provided the copper catalyst is filtered before zinc iodide is added.Prevailing mechanism
The reaction mechanism was first investigated by Scott Searles and coworkers at theUniversity of Missouri
The University of Missouri (Mizzou, MU, or Missouri) is a public university, public Land-grant university, land-grant research university in Columbia, Missouri. It is Missouri's largest university and the flagship of the four-campus Universit ...
. Overall, the reaction can be thought of as a reductive coupling of the carbonyl compound and the terminal alkyne. In the Crabbé reaction, the secondary amine serves as the hydride donor, which results in the formation of the corresponding imine as the byproduct. Thus, remarkably, the secondary amine serves as Brønsted base, ligand for the metal ion, iminium-forming carbonyl activator, and the aforementioned two-electron reductant in the same reaction.
In broad strokes, the mechanism of the reaction is believed to first involve a Mannich-like addition of the alkynylmetal species into the iminium ion formed by condensation of the aldehyde and the secondary amine. This first part of the process is a so-called A3 coupling reaction (A3 stands for aldehyde-alkyne-amine). In the second part, the α-amino alkyne then undergoes a formal retro-imino-ene reaction, an internal redox process, to deliver the desired allene and an imine as the oxidized byproduct of the secondary amine. These overall steps are supported by deuterium labeling and kinetic isotope effect studies. Density functional theory computations were performed to better understand the second part of the reaction. These computations indicate that the uncatalyzed process (either a concerted but highly asynchronous process or a stepwise process with a fleeting intermediate) involves a prohibitively high-energy barrier. The metal-catalyzed reaction, on the other hand, is energetically reasonable and probably occurs via a stepwise hydride transfer to the alkyne followed by C–N bond scission in a process similar to those proposed for formal ,3sigmatropic rearrangements and hydride transfer reactions catalyzed by gold(I) complexes. A generic mechanism showing the main features of the reaction (under Crabbé's original conditions) is given below: (The copper catalyst is shown simply as "CuBr" or "Cu+", omitting any additional amine or halide ligands or the possibility of dinuclear interactions with other copper atoms. Condensation of formaldehyde and diisopropylamine to form the iminium ion and steps involving complexation and decomplexation of Cu+ are also omitted here for brevity.)
Since 2012, Ma has reported several catalytic enantioselective versions of the Crabbé reaction in which chiral PINAP (aza-BINAP) based ligands for copper are employed. The stepwise application of copper and zinc catalysis was required: the copper promotes the Mannich-type condensation, while subsequent one-step addition of zinc iodide catalyzes the imino-retro-ene reaction.
(The copper catalyst is shown simply as "CuBr" or "Cu+", omitting any additional amine or halide ligands or the possibility of dinuclear interactions with other copper atoms. Condensation of formaldehyde and diisopropylamine to form the iminium ion and steps involving complexation and decomplexation of Cu+ are also omitted here for brevity.)
Since 2012, Ma has reported several catalytic enantioselective versions of the Crabbé reaction in which chiral PINAP (aza-BINAP) based ligands for copper are employed. The stepwise application of copper and zinc catalysis was required: the copper promotes the Mannich-type condensation, while subsequent one-step addition of zinc iodide catalyzes the imino-retro-ene reaction.
See also
*Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). ...
* Ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
* Coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
* Alkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an Alpha and beta carbon, α-alkynyl alcohol (chemistry), alcohol ().
When the acetylide is formed from acetylene () ...
References
{{Organic chemistry Organic chemistry Name reactions