Copper hydride on:
[Wikipedia]
[Google]
[Amazon]
Copper hydride is
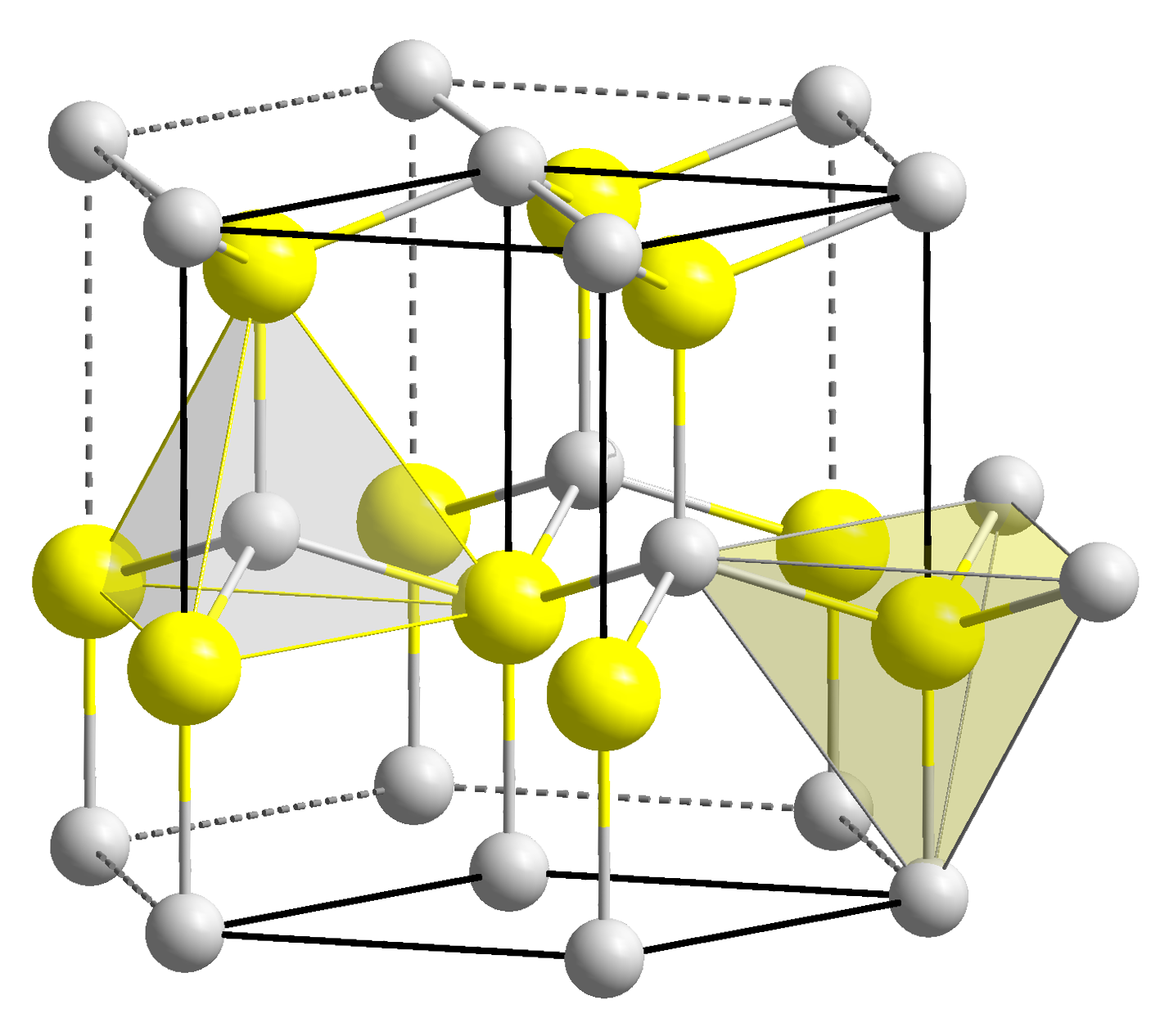 In copper hydride, elements adopt the Wurtzite crystal structure (
In copper hydride, elements adopt the Wurtzite crystal structure (
 Phosphine- and NHC-copper hydride species have been developed as reagents in organic synthesis, albeit of limited use. Most widely used is Ph3P)CuHsub>6 ( Stryker's reagent) for the reduction of α,β-unsaturated carbonyl compounds. H2 (at least 80 psi) and
Phosphine- and NHC-copper hydride species have been developed as reagents in organic synthesis, albeit of limited use. Most widely used is Ph3P)CuHsub>6 ( Stryker's reagent) for the reduction of α,β-unsaturated carbonyl compounds. H2 (at least 80 psi) and
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.
History
In 1844, the French chemistAdolphe Wurtz
Charles Adolphe Wurtz (; 26 November 181710 May 1884) was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinio ...
synthesised copper hydride for the first time. He reduced an aqueous solution of copper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hy ...
with hypophosphorous acid
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane
and alcohols. The formula for this ...
(H3PO2). In 2011, Panitat Hasin and Yiying Wu were the first to synthesise a metal hydride (copper hydride) using the technique of sonication. Copper hydride has the distinction of being the first metal hydride discovered. In 2013, it was established by Donnerer et al. that, at least up to fifty gigapascals, copper hydride cannot be synthesised by pressure alone. However, they were successful in synthesising several copper-hydrogen alloys under pressure.
Chemical properties
Structure
 In copper hydride, elements adopt the Wurtzite crystal structure (
In copper hydride, elements adopt the Wurtzite crystal structure (polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ic), being connected by covalent bonds.
The CuH consists of a core of CuH with a shell of water and this may be largely replaced by ethanol. This offers the possibility of modifying the properties of CuH produced by aqueous routes. While all methods for the synthesis of CuH result in the same bulk product, the synthetic path taken engenders differing surface properties. The different behaviors of CuH obtained by aqueous and nonaqueous routes can be ascribed to a combination of very different particle size and dissimilar surface termination, namely, bonded hydroxyls for the aqueous routes and a coordinated donor for the nonaqueous routes.
Chemical reactions
CuH generally behaves as a source of H–. For instance, Wurtz reported the double displacement reaction of CuH with hydrochloric acid: :CuH + HCl → CuCl + When not cooled below , copper hydride decomposes, to produce hydrogen gas and a mixture containing elemental copper: :2 CuH → ''x''Cu•(2-''x'')CuH + ½''x'' (0 < ''x'' < 2) Solid copper hydride is the irreversible autopolymerisation product of the molecular form, and the molecular form cannot be isolated in concentration.Production
Copper does not react with hydrogen even on heating, thus copper hydrides are made indirectly from copper(I) and copper(II) precursors. Examples include the reduction ofcopper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hy ...
with sodium hypophosphite
Sodium hypophosphite (NaPO2H2, also known as sodium phosphinate) is the sodium salt of hypophosphorous acid and is often encountered as the monohydrate, NaPO2H2·H2O. It is a solid at room temperature, appearing as odorless white crystals. It is so ...
in the presence of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
, or more simply with just hypophosphorous acid
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane
and alcohols. The formula for this ...
. Other reducing agents, including classical aluminium hydrides can be used.
:4 Cu2+ + 6 H3PO2 + 6 H2O → 4 CuH + 6 H3PO3 + 8 H+
The reactions produce a red-colored precipitate of CuH, which is generally impure and slowly decomposes to liberate hydrogen, even at 0 °C.
:2 CuH → 2 Cu + H2
This slow decomposition also takes place underwater, however there are reports of the material becoming pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
if dried.
A new synthesis method has been published in 2017 by Lousada et al. In this synthesis high purity CuH nanoparticles have been obtained from basic copper carbonate, CuCO3·Cu(OH)2. This method is faster and has a higher chemical yield than the copper sulfate based synthesis and produces nanoparticles of CuH with higher purity and a smaller size distribution. The obtained CuH can easily be converted to conducting thin films of Cu. These films are obtained by spraying the CuH nanoparticles in their synthesis medium into some insulating support. After drying, conducting Cu films protected by a layer of mixed copper oxides are spontaneously formed.
Reductive sonication
Copper hydride is also produced by reductive sonication. In this process, hexaaquacopper(II) and hydrogen(•) react to produce copper hydride and oxonium according to the equation: : u(H2O)6sup>2+ + 3 H• → 1/''n'' (CuH)''n'' + 2 3Osup>+ + 4 H2O Hydrogen(•) is obtained ''in situ'' from the homolytic sonication of water. Reductive sonication produces molecular copper hydride as an intermediate.Applications in Organic Synthesis
 Phosphine- and NHC-copper hydride species have been developed as reagents in organic synthesis, albeit of limited use. Most widely used is Ph3P)CuHsub>6 ( Stryker's reagent) for the reduction of α,β-unsaturated carbonyl compounds. H2 (at least 80 psi) and
Phosphine- and NHC-copper hydride species have been developed as reagents in organic synthesis, albeit of limited use. Most widely used is Ph3P)CuHsub>6 ( Stryker's reagent) for the reduction of α,β-unsaturated carbonyl compounds. H2 (at least 80 psi) and hydrosilane
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5 ...
s can be used as the terminal reductant, allowing a catalytic amount of Ph3P)CuHsub>6 to be used for conjugate reduction reactions.
Chiral phosphine-copper complexes catalyze hydrosilation of ketones and esters with low enanotioselectivities. An enantioselective (80 to 92% ee) reduction of prochiral α,β-unsaturated esters uses Tol-BINAP complexes of copper in the presence of PMHS as the reductant. Subsequently, conditions have been developed for the CuH-catalyzed hydrosilylation of ketones and imines proceeding with excellent levels of chemo- and enantioselectivity.
The reactivity of L''n''CuH species with weakly activated (e.g. styrenes, dienes) and unactivated alkenes (e.g. α-olefins) and alkynes has been recognized and has served as the basis for several copper-catalyzed formal hydrofunctionalization reactions.
"Hydridocopper"
The diatomic species CuH is a gas that has attracted the attention of spectroscopists. It polymerises upon being condensed. A well-known oligomer is ''octahedro''-hexacuprane(6), occurring in Stryker's reagent. Hydridocopper has acidic behavior for the same reason as normal copper hydride. However, it does not form stable aqueous solutions, due in part to its autopolymerisation, and its tendency to be oxidised by water. Copper hydride reversibly precipitates from pyridine solution, as an amorphous solid. However, repeated dissolution affords the regular crystalline form, which is insoluble. Under standard conditions, molecular copper hydride autopolymerises to form the crystalline form, including under aqueous conditions, hence the aqueous production method devised by Wurtz.Production
Molecular copper hydride can be formed by reducingcopper iodide
Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
Copper(I) iodide is white, but samples often appear ...
with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
in ether and pyridine. 4CuI + LiAlH4 → CuH + LiI + AlI3 This was discovered by E Wiberg and W Henle in 1952. The solution of this CuH in the pyridine is typically dark red to dark orange. A precipitate is formed if ether is added to this solution. This will redissolve in pyridine. Impurities of the reaction products remain in the product. In this study, it was found that the solidified diatomic substance is distinct from the Wurtzite structure. The Wurtzite substance was insoluble and was decomposed by lithium iodide, but not the solidified diatomic species. Moreover, while the Wurtzite substance's decomposition is strongly base catalysed, whereas the solidified diatomic species is not strongly affected at all. Dilts distinguishes between the two copper hydrides as the 'insoluble-' and 'soluble copper hydrides'. The soluble hydride is susceptible to pyrolysis under vacuum and proceeds to completion under 100 °C.
Amorphous copper hydride is also produced by anhydrous reduction. In this process copper(I) and tetrahydroaluminate react to produce molecular copper hydride and triiodoaluminium adducts. The molecular copper hydride is precipitated into amorphous copper hydride with the addition of diethyl ether. Amorphous copper hydride is converted into the Wurtz phase by annealing, accompanied by some decomposition.
History
Hydridocopper was discovered in the vibration-rotation emission of ahollow-cathode lamp
A hollow-cathode lamp (HCL) is type of cold cathode lamp used in physics and chemistry as a spectral line source (e.g. for atomic absorption spectrometers) and as a frequency tuner for light sources such as lasers. An HCL takes advantage of the ...
in 2000 by Bernath, who detected it at the University of Waterloo. It was first detected as a contaminant while attempting to generate NeH+ using the hollow-cathode lamp. Molecular copper hydride has the distinction of being the first metal hydride to be detected in this way. (1,0) (2,0) and (2,1) vibrational bands were observed along with line splitting due to the presence of two copper isotopes, 63Cu and 65Cu.
The A1Σ+-X1Σ+ absorption lines from CuH have been claimed to have been observed in sunspots and in the star 19 Piscium
TX Piscium (19 Piscium) is a variable carbon star in the constellation Pisces. It is amongst the reddest naked eye stars, with a significant reddish hue when seen in binoculars. It is approximately 900 light years from Earth.
Spectrum ...
.
In vapour experiments, it was found that copper hydride is produced from the elements upon exposure to 310 nanometre radiation.
:Cu + H2 ↔ CuH + H•
However, this proved to be unviable as a production method as the reaction is difficult to control. The activation barrier for the reverse reaction is virtually non-existent, which allows it to readily proceed even at 20 Kelvin.
Other copper hydrides
* A binary dihydride () also exists, in the form of an unstablereactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
in the reduction of copper hydride by atomic hydrogen.
References
{{Hydrides by group Copper(I) compounds Metal hydrides Wurtzite structure type