Chain Walking on:
[Wikipedia]
[Google]
[Amazon]
In
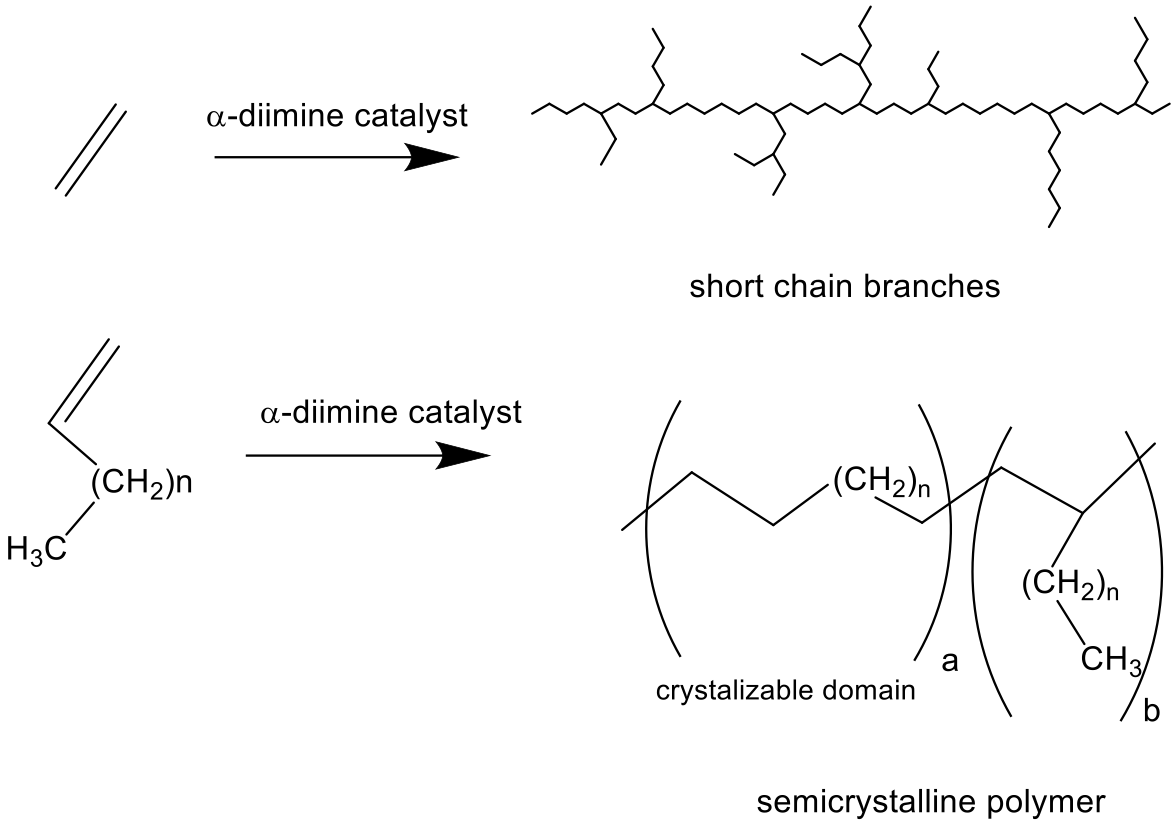 CW occurs after the polymer chain has grown somewhat on the metal catalyst. The precursor is a 16 e− complex with the general formula L2(C2H4)(chain)sup>+. The
CW occurs after the polymer chain has grown somewhat on the metal catalyst. The precursor is a 16 e− complex with the general formula L2(C2H4)(chain)sup>+. The  This process, a step in the chain walk, moves the metal from the end of a chain to a secondary carbon center. At this stage, two options are available: (1) chain walking can continue or (2) a molecule of ethylene can bind to reform the 16e complex. At this second resting state, the ethylene molecule can insert to grow the polymer or dissociate inducing further chain walking. If many branches can form, a hyperbranched topology results. Therefore, ethene only homopolymerization can provide branched polymer whereas the same mechanism leads to chain straightening in α-olefin polymerization. The variation of CW by changing T, monomer concentration, or catalyst switch can be used to produce block copolymer with amorphous and semi-crystalline blocks or with blocks of different topology.
This process, a step in the chain walk, moves the metal from the end of a chain to a secondary carbon center. At this stage, two options are available: (1) chain walking can continue or (2) a molecule of ethylene can bind to reform the 16e complex. At this second resting state, the ethylene molecule can insert to grow the polymer or dissociate inducing further chain walking. If many branches can form, a hyperbranched topology results. Therefore, ethene only homopolymerization can provide branched polymer whereas the same mechanism leads to chain straightening in α-olefin polymerization. The variation of CW by changing T, monomer concentration, or catalyst switch can be used to produce block copolymer with amorphous and semi-crystalline blocks or with blocks of different topology.

polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
, chain walking (CW) or chain running or chain migration is a mechanism that operates during some alkene polymerization
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
reactions. CW can be also considered as a specific case of intermolecular chain transfer (analogous to radical ethene polymerization). This reaction gives rise to branched and hyperbranched/dendritic hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The extent of CW, displayed in the number of branches formed and positions of branches on the polymers are controlled by the choice of a catalyst. The potential applications of polymers formed by this reaction are diverse, from drug delivery to phase transfer agents, nanomaterials, and catalysis.
Catalysts
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s that promote chain walking were discovered in the 1980-1990s. Nickel(II) and palladium(II) complexes of α-diimine ligand
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions ...
s were known to efficiently catalyze polymerization of alkenes. They are also referred as Brookhart's catalysts after being used for making of high molar mass polyolefins for the first time at University of North Carolina at Chapel Hill in 1995. Currently nickel and palladium complexes bearing α-diimine ligands, such as the two examples shown, are the most thoroughly described chain walking catalysts in scientific literature. Ligand design influences not only CW extent but also regio- and stereoselectivity and also the sensitivity of the catalyst to undergo chain-breaking reactions, mainly β-H elimination, influencing achievable molar mass and also the possibility to achieve living polymerization behaviour. Thus stereo block copolymers could be made by combination of living and stereospecific CW polymerization catalysts. Continuous research effort led to design of other ligands which provide CW polymerization catalysts upon complexation to late transition metals. Examples are β-diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions ...
, α-keto-β-diimine, amine-imine and most recently diamine ligands. As the vast majority of CW polymerization catalysts is based on late-transition metal complexes, having generally lower oxophilicity, these complexes were demonstrated also to provide copolymerisation of olefins with polar monomers like acrylates, alkylvinylketones, ω-alken-1-ols, ω-alken-1-carboxylic acids etc., which was the main initial intention of development of this class of catalysts. These random copolymers could further be utilized in the construction of sophisticated amphiphilic grafted copolymers with hydrophobic polyolefin core and shell based on hydrophilic arms, in some cases made of stimuli-responsive polymers.
Mechanism
 CW occurs after the polymer chain has grown somewhat on the metal catalyst. The precursor is a 16 e− complex with the general formula L2(C2H4)(chain)sup>+. The
CW occurs after the polymer chain has grown somewhat on the metal catalyst. The precursor is a 16 e− complex with the general formula L2(C2H4)(chain)sup>+. The ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
(the monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
) dissociates to produce a highly unsaturated 14 e− cation. This cation is stabilized by an agostic interaction
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, r ...
. β-Hydride elimination then occurs to give a hydride-alkene complex. Subsequent reinsertion of the M-H into the C=C bond, but in the opposite sense gives a metal-alkyl complex.
 This process, a step in the chain walk, moves the metal from the end of a chain to a secondary carbon center. At this stage, two options are available: (1) chain walking can continue or (2) a molecule of ethylene can bind to reform the 16e complex. At this second resting state, the ethylene molecule can insert to grow the polymer or dissociate inducing further chain walking. If many branches can form, a hyperbranched topology results. Therefore, ethene only homopolymerization can provide branched polymer whereas the same mechanism leads to chain straightening in α-olefin polymerization. The variation of CW by changing T, monomer concentration, or catalyst switch can be used to produce block copolymer with amorphous and semi-crystalline blocks or with blocks of different topology.
This process, a step in the chain walk, moves the metal from the end of a chain to a secondary carbon center. At this stage, two options are available: (1) chain walking can continue or (2) a molecule of ethylene can bind to reform the 16e complex. At this second resting state, the ethylene molecule can insert to grow the polymer or dissociate inducing further chain walking. If many branches can form, a hyperbranched topology results. Therefore, ethene only homopolymerization can provide branched polymer whereas the same mechanism leads to chain straightening in α-olefin polymerization. The variation of CW by changing T, monomer concentration, or catalyst switch can be used to produce block copolymer with amorphous and semi-crystalline blocks or with blocks of different topology.

References
{{reflist Polymer chemistry Organometallic chemistry