Cationic Polymer on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, cationic polymerization is a type of chain growth polymerization in which a

 Heterocyclic monomers that are cationically polymerized are
Heterocyclic monomers that are cationically polymerized are





 The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.
The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.


 Butyl rubber, in contrast to PIB, is a copolymer in which the monomers
Butyl rubber, in contrast to PIB, is a copolymer in which the monomers
cationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
initiator transfers charge to a monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
which then becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer.
The types of monomers necessary for cationic polymerization are limited to alkenes with electron-donating substituents and heterocycles. Similar to anionic polymerization
In polymer chemistry, anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but tradi ...
reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain.
Cationic polymerization is used in the production of polyisobutylene
Polyisobutene (polyisobutylene) is a class of organic polymers prepared by polymerization of isobutene. The polymers often have the formula Me3C H2CMe2sub>nX (Me = CH3, X = H, F). They are typically colorless gummy solids.
Polymerization is typ ...
(used in inner tubes) and poly( N-vinylcarbazole) (PVK).
Monomers
Monomer scope for cationic polymerization is limited to two main types:alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
and heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
monomers. Cationic polymerization of both types of monomers occurs only if the overall reaction is thermally favorable. In the case of alkenes, this is due to isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
of the monomer double bond; for heterocycles, this is due to release of monomer ring strain and, in some cases, isomerization of repeating units. Monomers for cationic polymerization are nucleophilic and form a stable cation upon polymerization.
Alkenes
Cationic polymerization of olefin monomers occurs with olefins that contain electron-donating substituents. These electron-donating groups make the olefinnucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
enough to attack electrophilic initiators or growing polymer chains. At the same time, these electron-donating groups attached to the monomer must be able to stabilize the resulting cationic charge for further polymerization. Some reactive olefin monomers are shown below in order of decreasing reactivity, with heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
groups being more reactive than alkyl or aryl groups. Note, however, that the reactivity of the carbenium ion formed is the opposite of the monomer reactivity. 
Heterocyclic monomers
 Heterocyclic monomers that are cationically polymerized are
Heterocyclic monomers that are cationically polymerized are lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s, lactams and cyclic amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s. Upon addition of an initiator, cyclic monomers go on to form linear polymers. The reactivity of heterocyclic monomers depends on their ring strain. Monomers with large ring strain, such as oxirane
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly swe ...
, are more reactive than 1,3-dioxepane which has considerably less ring strain. Rings that are six-membered and larger are less likely to polymerize due to lower ring strain.
Synthesis
Initiation
Initiation is the first step in cationic polymerization. During initiation, acarbenium ion
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
is generated from which the polymer chain is made. The counterion should be non-nucleophilic, otherwise the reaction is terminated instantaneously. There are a variety of initiators available for cationic polymerization, and some of them require a coinitiator to generate the needed cationic species.
Classical protic acids
Strongprotic
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
acids can be used to form a cationic initiating species. High concentrations of the acid are needed in order to produce sufficient quantities of the cationic species. The counterion (A−) produced must be weakly nucleophilic so as to prevent early termination due to combination with the protonated alkene. Common acids used are phosphoric, sulfuric, fluoro-, and triflic acids. Only low molecular weight polymers are formed with these initiators.

Lewis acids/Friedel-Crafts catalysts
Lewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any spe ...
are the most common compounds used for initiation of cationic polymerization. The more popular Lewis acids are SnCl4, AlCl3, BF3, and TiCl4. Although these Lewis acids alone are able to induce polymerization, the reaction occurs much faster with a suitable cation source. The cation source can be water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, alcohols, or even a carbocation donor such as an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
or an anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
. In these systems the Lewis acid is referred to as a coinitiator while the cation source is the initiator. Upon reaction of the initiator with the coinitiator, an intermediate complex is formed which then goes on to react with the monomer unit. The counterion produced by the initiator-coinitiator complex is less nucleophilic than that of the Brønsted acid A− counterion. Halogens, such as chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
and bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
, can also initiate cationic polymerization upon addition of the more active Lewis acids.

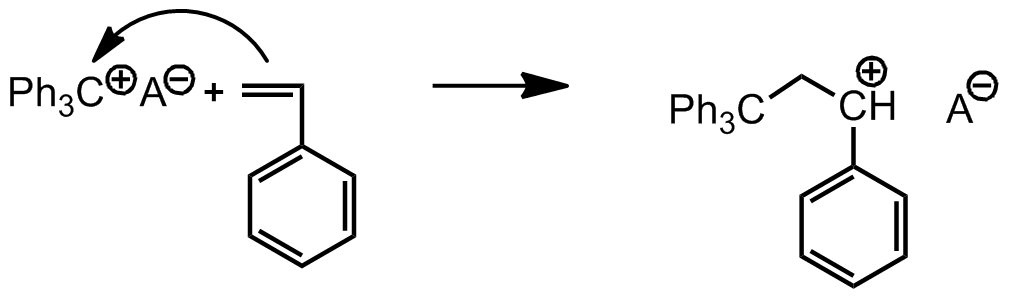
Carbenium ion salts
Stable carbenium ions are used to initiate chain growth of only the most reactive alkenes and are known to give well defined structures. These initiators are most often used in kinetic studies due to the ease of measuring the disappearance of the carbenium ion absorbance. Common carbenium ions aretrityl
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmetha ...
and tropylium
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of ...
cations.

Ionizing radiation
Ionizing radiation can form a radical-cation pair that can then react with a monomer to start cationic polymerization. Control of the radical-cation pairs is difficult and often depends on the monomer and reaction conditions. Formation of radical and anionic species is often observed.
Propagation
Propagation proceeds by addition of monomer to the active species, i.e. the carbenium ion. The monomer is added to the growing chain in a head-to-tail fashion; in the process, the cationic end group is regenerated to allow for the next round of monomer addition.
Effect of temperature
The temperature of the reaction has an effect on the rate of propagation. The overall activation energy for the polymerization () is based upon the activation energies for the initiation (), propagation (), and termination () steps: : Generally, is larger than the sum of and , meaning the overallactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
is negative. When this is the case, a decrease in temperature leads to an increase in the rate of propagation. The converse is true when the overall activation energy is positive.
Chain length is also affected by temperature. Low reaction temperatures, in the range of 170–190 K, are preferred for producing longer chains. This comes as a result of the activation energy for termination and other side reactions being larger than the activation energy for propagation. As the temperature is raised, the energy barrier for the termination reaction is overcome, causing shorter chains to be produced during the polymerization process.
Effect of solvent and counterion
The solvent and the counterion (the gegen ion) have a significant effect on the rate of propagation. The counterion and the carbenium ion can have different associations according tointimate ion pair
In chemistry, the intimate ion pair concept, introduced by Saul Winstein, describes the interactions between a cation, anion and surrounding solvent molecules. In ordinary aqueous solutions of inorganic salts, an ion is completely solvated and sh ...
theory; ranging from a covalent bond, tight ion pair (unseparated), solvent-separated ion pair (partially separated), and free ions (completely dissociated).
The association is strongest as a covalent bond and weakest when the pair exists as free ions. In cationic polymerization, the ions tend to be in equilibrium between an ion pair (either tight or solvent-separated) and free ions. The more polar the solvent used in the reaction, the better the solvation and separation of the ions. Since free ions are more reactive than ion pairs, the rate of propagation is faster in more polar solvents.
The size of the counterion is also a factor. A smaller counterion, with a higher charge density, will have stronger electrostatic interactions with the carbenium ion than will a larger counterion which has a lower charge density. Further, a smaller counterion is more easily solvated by a polar solvent than a counterion with low charge density. The result is increased propagation rate with increased solvating capability of the solvent.
Termination
Termination generally occurs by unimolecular rearrangement with the counterion. In this process, an anionic fragment of the counterion combines with the propagating chain end. This not only inactivates the growing chain, but it also terminates the kinetic chain by reducing the concentration of the initiator-coinitiator complex.
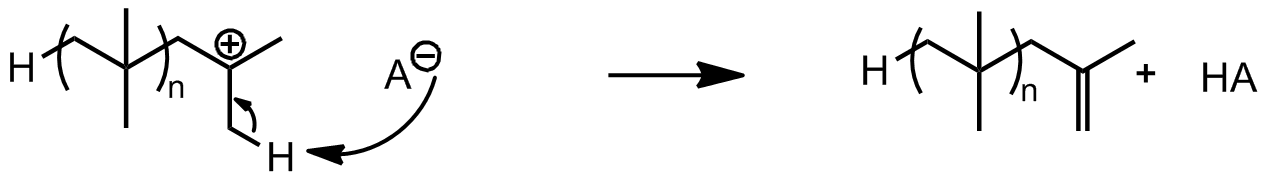
Chain transfer
Chain transfer can take place in two ways. One method of chain transfer is hydrogen abstraction from the active chain end to the counterion. In this process, the growing chain is terminated, but the initiator-coinitiator complex is regenerated to initiate more chains. The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.
The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.

Cationic ring-opening polymerization
Cationicring-opening polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anion ...
follows the same mechanistic steps of initiation, propagation, and termination. However, in this polymerization reaction, the monomer units are cyclic in comparison to the resulting polymer chains which are linear. The linear polymers produced can have low ceiling temperature Ceiling temperature (T_c) is a measure of the tendency of a polymer to revert to its constituent monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the cei ...
s, hence end-capping of the polymer chains is often necessary to prevent depolymerization.

Kinetics
The rate of propagation and the degree of polymerization can be determined from an analysis of the kinetics of the polymerization. The reaction equations for initiation, propagation, termination, and chain transfer can be written in a general form: : In which I+ is the initiator, M is the monomer, M+ is the propagating center, and , , , and are the rate constants for initiation, propagation, termination, and chain transfer, respectively. For simplicity, counterions are not shown in the above reaction equations and only chain transfer to monomer is considered. The resulting rate equations are as follows, where brackets denote concentrations: : Assuming steady-state conditions, i.e. the rate of initiation = rate of termination: : This equation for +can then be used in the equation for the rate of propagation: : From this equation, it is seen that propagation rate increases with increasing monomer and initiator concentration. Thedegree of polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given b ...
, , can be determined from the rates of propagation and termination:
:
If chain transfer rather than termination is dominant, the equation for becomes
:
Living polymerization
In 1984, Higashimura and Sawamoto reported the first living cationic polymerization for alkyl vinyl ethers. This type of polymerization has allowed for the control of well-defined polymers. A key characteristic of living cationic polymerization is that termination is essentially eliminated, thus the cationic chain growth continues until all monomer is consumed.Commercial applications
The largest commercial application of cationic polymerization is in the production of polyisobutylene (PIB) products which includepolybutene Polybutene is an organic polymer made from a mixture of 1-butene, 2-butene, and isobutylene. Ethylene steam cracker C4s are also used as supplemental feed for polybutene. It is similar to polyisobutylene (PIB), which is produced from essentially ...
and butyl rubber
Butyl rubber, sometimes just called "butyl", is a synthetic rubber, a copolymer of isobutylene with isoprene. The abbreviation IIR stands for isobutylene isoprene rubber. Polyisobutylene, also known as "PIB" or polyisobutene, (C4H8)n, is the ho ...
. These polymers have a variety of applications from adhesives and sealants to protective gloves and pharmaceutical stoppers. The reaction conditions for the synthesis of each type of isobutylene product vary depending on the desired molecular weight and what type(s) of monomer(s) is used. The conditions most commonly used to form low molecular weight (5–10 x 104 Da) polyisobutylene are initiation with AlCl3, BF3, or TiCl4 at a temperature range of −40 to 10 °C. These low molecular weight polyisobutylene polymers are used for caulking and as sealants. High molecular weight PIBs are synthesized at much lower temperatures of −100 to −90 °C and in a polar medium of methylene chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
. These polymers are used to make uncrosslinked rubber products and are additives for certain thermoplasts. Another characteristic of high molecular weight PIB is its low toxicity which allows it to be used as a base for chewing gum. The main chemical companies that produce polyisobutylene are Esso, ExxonMobil, and BASF
BASF SE () is a German multinational chemical company and the largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany.
The BASF Group comprises subsidiaries and joint ventures in more than 80 countries ...
.
isobutylene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Producti ...
(~98%) and isoprene (2%) are polymerized in a process similar to high molecular weight PIBs. Butyl rubber polymerization is carried out as a continuous process with AlCl3 as the initiator. Its low gas permeability and good resistance to chemicals and aging make it useful for a variety of applications such as protective gloves, electrical cable insulation, and even basketballs. Large scale production of butyl rubber started during World War II, and roughly 1 billion pounds/year are produced in the U.S. today.
Polybutene is another copolymer, containing roughly 80% isobutylene and 20% other butenes (usually 1-butene). The production of these low molecular weight polymers (300–2500 Da) is done within a large range of temperatures (−45 to 80 °C) with AlCl3 or BF3. Depending on the molecular weight of these polymers, they can be used as adhesives, sealants, plasticizers, additives for transmission fluids, and a variety of other applications. These materials are low-cost and are made by a variety of different companies including BP Chemicals, Esso, and BASF.
Other polymers formed by cationic polymerization are homopolymers and copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
of polyterpenes, such as pinene
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major comp ...
s (plant-derived products), that are used as tackyfiers. In the field of heterocycles, 1,3,5-trioxane
1,3,5-Trioxane, sometimes also called trioxane or trioxin, is a chemical compound with molecular formula CHO. It is a white, highly water-soluble solid with a chloroform-like odor. It is a stable cyclic trimer of formaldehyde, and one of the th ...
is copolymerized with small amounts of ethylene oxide to form the highly crystalline polyoxymethylene plastic. Also, the homopolymerization of alkyl vinyl ethers is achieved only by cationic polymerization.
References
{{Reflist, 30em Polymerization reactions