Carroll Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
The Carroll rearrangement is a
 Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0):
Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0):

 A similar reaction uses additional
A similar reaction uses additional 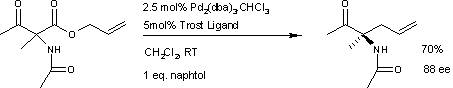 This reaction delivers the main
This reaction delivers the main  The scope is extended to asymmetric α-alkylation of
The scope is extended to asymmetric α-alkylation of 
rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
and involves the transformation of a β- keto allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
into a α-allyl-β-ketocarboxylic acid. This organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
is accompanied by decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a ,3sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, ...
and effectively a decarboxylative allylation.
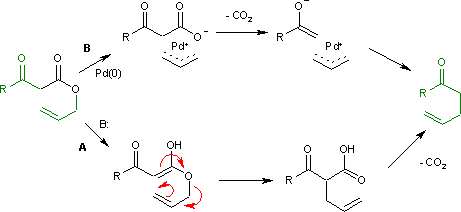
Reaction mechanism
The Carroll rearrangement (1940) in the presence of base and with high reaction temperature (path A) takes place through an intermediateenol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
which then rearranges in an electrocyclic Claisen rearrangement. The follow-up is a decarboxylation. With palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
(0) as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, the reaction (Tsuji, 1980) is much milder (path B) with an intermediate allyl cation / carboxylic acid anion organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
complex.
 Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0):
Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0):

Asymmetric decarboxylative allylation
By introducing suitable chiral ligands, the reaction becomesenantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
.
The first reported asymmetric
Asymmetric may refer to:
*Asymmetry in geometry, chemistry, and physics
Computing
* Asymmetric cryptography, in public-key cryptography
*Asymmetric digital subscriber line, Internet connectivity
* Asymmetric multiprocessing, in computer architect ...
rearrangement is catalyzed by tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or d2(dba)3is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Beca ...
and the Trost ligand
The Trost ligand is a diphosphine used in the palladium-catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from ''trans''-1,2-diaminocyclohexane (DACH) have been developed, such as the (''R'',''R'')-DACH-naphthyl l ...
:''Asymmetric Allylic Alkylation of Ketone Enolates: An Asymmetric Claisen Surrogate'' Erin C. Burger and Jon A. Tunge Org. Lett.; 2004; 6(22) pp 4113 - 4115; (Letter)
 A similar reaction uses additional
A similar reaction uses additional naphthol Naphthol may refer to:
* 1-Naphthol
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula . It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The ...
.
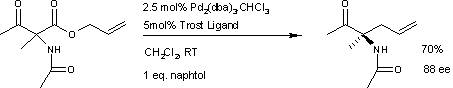 This reaction delivers the main
This reaction delivers the main enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
with 88% enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a s ...
. It remains to be seen if this reaction will have a wide scope because the acetamido group appears to be a prerequisite.
The same catalyst but a different ligand is employed in this enantioconvergent reaction:
 The scope is extended to asymmetric α-alkylation of
The scope is extended to asymmetric α-alkylation of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s masked as their enol carbonate ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they ...
s:''Palladium-Catalyzed Asymmetric Allylic α-Alkylation of Acyclic Ketones'' Barry M. Trost
Barry M. Trost (born June 13, 1941, in Philadelphia) is an American chemist who is the Job and Gertrud Tamaki Professor Emeritus in the School of Humanities and Sciences at Stanford University. The Tsuji-Trost reaction and the Trost ligand are ...
and Jiayi Xu
J. Am. Chem. Soc.
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical ...
; 2005; 127(49) pp 17180 - 17181; (Communication)

References
{{DEFAULTSORT:Carroll rearrangement Rearrangement reactions Palladium Name reactions