Carbones on:
[Wikipedia]
[Google]
[Amazon]
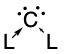 Carbones are a class of
Carbones are a class of
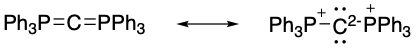 However, computational studies on
However, computational studies on
 Alternative methods to synthesise alkyl-substituted carbodiphosphoranes involve the
Alternative methods to synthesise alkyl-substituted carbodiphosphoranes involve the  Synthetic methods have also been developed for more diverse carbodiphosphoranes. Methylenediphosphines will undergo a reaction with
Synthetic methods have also been developed for more diverse carbodiphosphoranes. Methylenediphosphines will undergo a reaction with 
 Similar non-cyclic carbodicarbenes have also been successfully synthesised from
Similar non-cyclic carbodicarbenes have also been successfully synthesised from 
 Carbodicarbenes have also been shown to form complexes with different transition metals such as
Carbodicarbenes have also been shown to form complexes with different transition metals such as

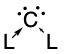 Carbones are a class of
Carbones are a class of molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
in the 1D excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
with a formal oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of zero where all four valence electrons
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
exist as unbonded lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s. These carbon-based compounds are of the formula CL2 where L is a strongly σ-donating ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
, typically a phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
(carbodiphosphoranes) or a N-heterocyclic carbene/NHC (carbodicarbenes), that stabilises the central carbon atom through donor-acceptor bonds. Carbones possess high-energy orbitals with both σ- and π-symmetry, making them strong Lewis bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any s ...
and strong π-backdonor substituents. Carbones possess high proton affinities and are strong nucleophiles
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
which allows them to function as ligands in a variety of main group
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arra ...
and transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
complexes. Carbone-coordinated elements also exhibit a variety of different reactivities and catalyse
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
various organic and main group reactions
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and me ...
.
Structure and bonding
Carbodiphosphoranes
In the initial syntheses of carbodiphosphoranes, the structure was described as aresonance hybrid
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
between an overall neutral species in which double bonds exists between the central carbon atom and the two complexed phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
atoms and a zwitterionic
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
species that places a positive charge on both phosphorus atoms and an overall charge of -2 on the central carbon atom.
 However, computational studies on
However, computational studies on hexaphenylcarbodiphosphorane
Hexaphenylcarbodiphosphorane is the organophosphorus compound with the formula C(PPh3)2 (where Ph = C6H5). It is a yellow, moisture-sensitive solid. The compound is classified as an ylide and as such carries significant negative charge on car ...
revealed that the highest-occupied molecular orbitals were both primarily localised on carbon and possessed shapes that were indicative of σ- and π-symmetric lone pairs rather than bonding molecular orbitals. Additional calculations showed σ-bonding orbitals between the central carbon atom and complexed phosphorus atoms but no orbitals localised on phosphorus, indicating the phosphorus atoms were donating their lone pairs into unoccupied valence orbitals on carbon to form a donor-acceptor complex. Crystallographic
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word ...
data also revealed that the hexaphenylcarbodiphosphorane structure was noticeably bent rather than linear with a P-C-P bond angle of 131.7°. Carbodicarbenes
The structure of carbodicarbenes greatly resembles that of carbodiphosphoranes. Computational data for a N-methyl-substituted carbodicarbene predicted a carbon-carbon bond with a length only marginally longer than a C=C bond in a typicalallene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which ...
at 1.358 Å (compared with 1.308 Å for allene), but with a significantly bent bond angle of 131.8° (compared to 180° for a standard linear allene). X-ray crystallography confirmed the structure with an experimentally-measured C=C bond length of 1.348 Å and a C-C-C bond angle of 131.8° indicative of two lone pairs present on the central carbon atom. Further calculations revealed the two highest-occupied molecular orbitals to be primarily localised on the central carbon atom as two lone pairs, like with the hexaphenylcarbodiphosphorane, albeit with slightly more delocalisation of the π-symmetric orbital onto the N-heterocyclic carbene carbon atoms due to their improved π-accepting properties. This is suggestive of a donor-acceptor interaction between the N-heterocyclic carbene ligands and a formally carbon (0) atom with two free lone pairs.Other carbene structures
Phosphaketene ylides (general formula R3P=C=C=O) andcarbon suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , whi ...
(O=C=C=C=O) have also exhibited carbone-like character where a carbon (0) species participates in a donor-acceptor interaction with carbon monoxide. The crystal structure of triphenylphosphoranylideneketen (Ph3PC2O) revealed a P-C-C bond angle of 145.5° consistent with the bent structure of other carbon (0) compounds. While both computational and experimental data indicated a linear structure for carbon suboxide, the same models predicted only an energy difference of 1.9 kcal mol-1 (7.9 kJ mol-1) between linear carbon suboxide and bent carbon suboxide. The ease of bending and relatively large contribution of carbon in the two highest-occupied molecular orbitals imply a certain degree of carbone-like character in spite of the linear geometry.
Synthesis
Carbodiphosphoranes
One strategy for the synthesis of carbodiphosphoranes involves the use of areducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth meta ...
on a carbon reagent in its +2 or +4 oxidation state. The first successful synthesis of a compound now recognised as a carbodiphosphorane was achieved by Ramirez et al. in 1961 with this method. By stirring methylidebis-(triphenylphosphonium) bromide with potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
metal suspended in a diglyme
Diglyme, or bis(2-methoxyethyl) ether, is a solvent with a high boiling point. It is an organic compound which is the dimethyl ether of diethylene glycol. (The name ''diglyme'' is a portmanteau of ''diglycol methyl ether''.) It is a colorless li ...
solution, the potassium reduced the starting material to form hexaphenylcarbodiphosphorane as a stable, yellow, crystalline solid.
 Alternative methods to synthesise alkyl-substituted carbodiphosphoranes involve the
Alternative methods to synthesise alkyl-substituted carbodiphosphoranes involve the deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
or elimination of carbon (IV) or carbon (II) starting materials. Reacting a carbon (IV) or carbon (II) diphosphine salt with a strong base such as sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
or sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white ...
can deprotonate the centre carbon atom to form the desired carbodiphosphorane. Alternatively, a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
-substituted phosphonium salt can undergo an elimination reaction in the presence of a strong base to form a carbodiphosphorane.
 Synthetic methods have also been developed for more diverse carbodiphosphoranes. Methylenediphosphines will undergo a reaction with
Synthetic methods have also been developed for more diverse carbodiphosphoranes. Methylenediphosphines will undergo a reaction with hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
or thioacetone
Thioacetone is an organosulfur compound belonging to the -thione group called thioketones, with a chemical formula (CH3)2CS. It is an unstable orange or brown substance that can be isolated only at low temperatures. Above , thioacetone readily co ...
to form O-substituted and S-substituted carbodiphosphoranes respectively. Cyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in s ...
carbodiphosphoranes have also been successfully synthesised through the reaction of bis(diisopropylamino)phosphino diazomethane with bis(dialkylamino)phosphenium triflate in excess benzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.
Production
It is p ...
followed by deprotonation with hexamethyldisilazide
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula CH3)3Sisub>2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An e ...
.

Carbodicarbenes
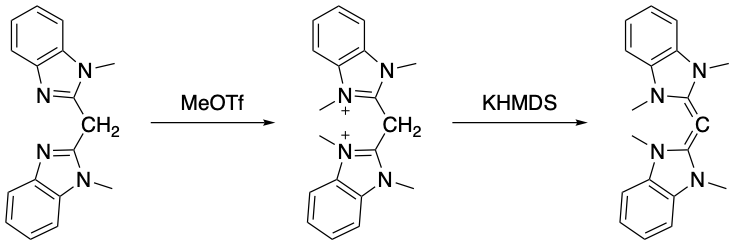
The first carbodicarbene synthesis was achieved much later than the first carbodiphosphorane synthesis, in 2008 by Dyker et al. The first step was themethylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
of bis(N-methylbenzimidazol-2-yl)methane using methyl triflate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. T ...
and the second step was the deprotonation of the carbon (II) species using potassium bis(trimethylsilyl)amide (KHMDS) to yield the desired N-heterocyclic-carbene-substituted carbone.
 Similar non-cyclic carbodicarbenes have also been successfully synthesised from
Similar non-cyclic carbodicarbenes have also been successfully synthesised from iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
salts through the condensation of two equivalents of the starting material in dimethylacetamide (DMA), followed by nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
with dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to aroun ...
, then deprotonation with n-butyllithium
''n''-Butyllithium C4H9Li (abbreviated ''n''-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly emp ...
to form a tetraaminoallene which acts as a carbodicarbene. Additionally, a method of facile synthesis of asymmetric carbodicarbenes was developed by Chen et al. in 2015 by using a simple nucleophilic substitution reaction. Reacting an olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
substituted with a N-heterocyclic carbene scaffold with a thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sul ...
containing a different NHC moiety generates a product which can be readily deprotonated to afford a carbodicarbene with two different carbene substituents with improved functionality.

Reactivity
Basicity
The presence of two lone pairs on the central carbon atom make it possible for carbones to act as Brønsted-Lowry bases and accept two protons from an acid. The typical first proton affinity for a carbodiphosphorane ranges from 209.3 kcal mol-1 (875.7 kJ mol-1) for the weakest base to 287.6 kcal mol-1 (1203 kJ mol-1) for the strongest base and second proton affinities ranging from 70.6 kcal mol-1 (295 kJ mol-1) to 188.9 kcal mol-1 (790.4 kJ mol-1). For comparison, the proton affinity ofpotassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
is 1101.8 kJ mol-1, indicating that carbodiphosphoranes can function as strong bases. Carbodicarbenes can act as even stronger bases than carbodiphosphoranes with first proton affinities reaching as high as 294.3 kcal mol-1 (1231 kJ mol-1). However, the second proton affinities for carbodicarbenes are comparable to those of carbodiphosphoranes and exhibit variability depending on the identity of the N-heterocyclic carbene substituent with a range of values from 155.3 kcal mol-1 (649.8 kJ mol-1) to 168.4 kcal mol-1 (704.6 kJ mol-1). This is due to the increased delocalisation of the π-symmetric lone pair over the carbon atoms of the N-heterocyclic carbene substituents which increases the dependence of the second proton affinity on the identity of the substituent.
Ligands
In addition to being strong Brønsted-Lowry bases, carbones are also nucleophilic and act as strong Lewis bases when coordinating with transition metals and main group elements. Several computational studies found that carbodiphosphoranes bound tightly totungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
with metal-ligand bond dissociation energies that were greater than those of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
metal-ligand bonds for certain compounds. Experimentally, a variety of metal-carbodiphosphorane complexes have been synthesised and characterised, including with metals such as tungsten, nickel, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, and gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
. The gold complex is of particular note as it is the first geminal
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to ...
digold complex and provides experimental evidence supporting the structure of carbodiphosphoranes as a carbon (0) compound with two lone pairs on the central carbon atom donating to the gold atoms.
 Carbodicarbenes have also been shown to form complexes with different transition metals such as
Carbodicarbenes have also been shown to form complexes with different transition metals such as rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
and gold. In the former experiment, when a rhodium carbonyl complex was coordinated to a carbodicarbene, the carbon-oxygen stretching frequency was observed at 2014 cm-1 which is significantly lower than the same carbon-oxygen stretching frequency when rhodium is coordinated to a N-heterocyclic carbene (between 2058 cm-1 and 2036 cm-1) which is indicative of a strong π-donating effect from the second carbon lone-pair of the carbone.
Reactivity in transition metal complexes
Transition metal complexes containing a carbone ligand also exhibit a wide range of reactivity. In 2015, Pranckevicius et al. synthesised aruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
(II) catalyst coordinated to two different carbodicarbene ligands that was able to catalytically reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox react ...
olefins with excellent diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
and similar activity to Crabtree’s catalyst. Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
(II) catalysts with bis(pyridine)carbodicarbene ligands have been shown to be successful catalysts for Suzuki-Miyaura and Heck-Mizoroki coupling reactions while rhodium (I) catalysts coordinated to carbodicarbene pincer ligands have been shown to hydroaminate and hydroarylate dienes Dienes may refer to:
* Dienes (surname), including a list of people with the name
* the plural of diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon at ...
.

Reactivity in main group complexes
Carbones can also form complexes with main group elements. The strong σ- and π-donating properties of carbones have made them optimal tools for stabilising reactive main-group-based species. Carbodicarbenes have been employed in the successful synthesis of novelboron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
-containing compounds such as borenium ions, which can exhibit useful optical properties, as well as a dicationic tricoordinate hydridoboron compound. Carbones have also been used in the first synthesis of stable carbon-bismuth
Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental ...
species with π-bonding character. Carbodicarbenes have also seen significant utility in the field of beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form mi ...
chemistry with the synthesis of a five-membered beryllacycle through C-H activation as well as beryllacycle ring expansion
Ring expansion and ring contraction reactions in the course of organic synthesis refer to a set of reactions which can lead to the expansion or contraction of an existing ring. This often makes it possible to access structures that would be dif ...
.

References
{{more categories, date=November 2022 Carbon compounds