Carbene radical on:
[Wikipedia]
[Google]
[Amazon]
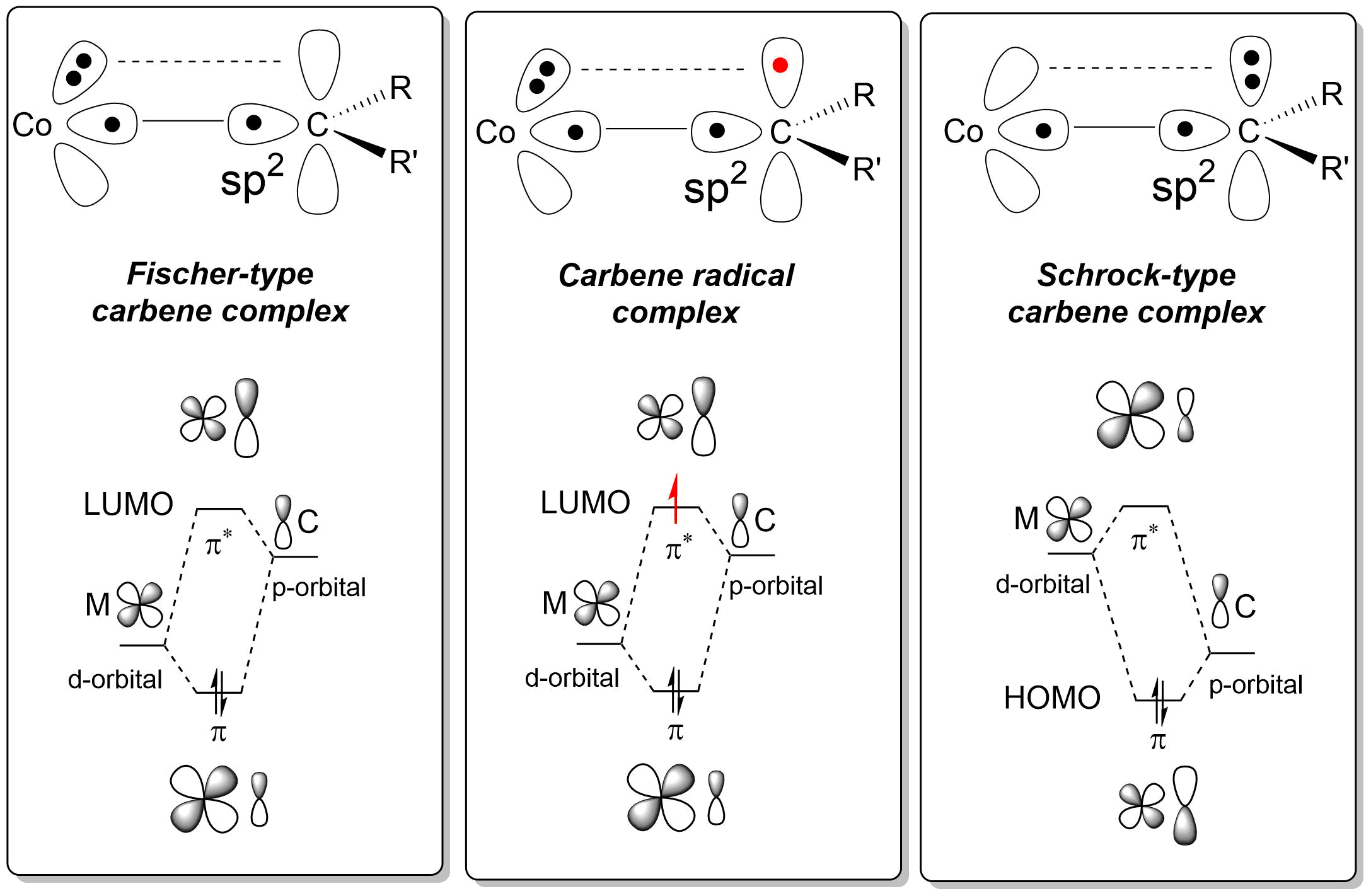 Carbene radicals are a special class of organometallic carbenes. The
Carbene radicals are a special class of organometallic carbenes. The 
 Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs, leads the typical radical character of the carbene carbon. This behaviour not only explains the carbon-centered radical-type reactivity of these complexes, but also their reduced electrophilicity (suppressing carbene-carbene dimerisation side reactions) as well as their enhanced reactivity to electron-deficient substrates. Furthermore, second coordination sphere hydrogen-bonding interactions give rise to faster reactions because H-bonds are stronger to the reduced carbene as compared to the precursor.
Such H-bonding interactions can also facilitate chirality transfer in enantioselective carbene-transfer reactions.
In order for the
Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs, leads the typical radical character of the carbene carbon. This behaviour not only explains the carbon-centered radical-type reactivity of these complexes, but also their reduced electrophilicity (suppressing carbene-carbene dimerisation side reactions) as well as their enhanced reactivity to electron-deficient substrates. Furthermore, second coordination sphere hydrogen-bonding interactions give rise to faster reactions because H-bonds are stronger to the reduced carbene as compared to the precursor.
Such H-bonding interactions can also facilitate chirality transfer in enantioselective carbene-transfer reactions.
In order for the
 Carbene radicals are a special class of organometallic carbenes. The
Carbene radicals are a special class of organometallic carbenes. The carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
can be formed by one-electron reduction of Fischer-type carbenes using an external reducing agent, or directly upon carbene formation at an open-shell transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
complex (in particular low-spin cobalt(II) complexes) using diazo compounds and related carbene precursors.
Cobalt(III)-carbene radicals have found catalytic applications in cyclopropanation reactions,
as well as in a variety of other catalytic radical-type ring-closing reactions.
Theoretical calculations and EPR studies confirmed their radical-type behaviour and explained the bonding interactions underlying the stability of the carbene radical.
Stable carbene radicals of other metals are known, but the catalytically relevant cobalt(III)-carbene radicals have thus far only been synthesized as long-lived reactive intermediates
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these com ...
.

Bonding interactions and radical reactivity
The chemical bond present in carbene radicals is surprising in that it possesses aspects of bothFischer
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher.
People with the surname A
* Abraham Fischer (1850–1913) South African public official
* Ad ...
and Schrock type carbenes. As a result, the cobalt carbene radical complexes have discrete radical-character at their carbon atom, thus giving rise to interesting catalytic radical-type reaction pathways.
The mechanism of formation of a carbene radical at cobalt(II) typically involves carbene generation at the metal with simultaneous intramolecular electron transfer from the metal into the metal-carbene π* anti-bonding
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more no ...
molecular orbital constructed from the metal d-orbital and the carbene p-orbital. As such, carbene radicals are perhaps best described as 'one-electron reduced Fischer-type carbenes'.  Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs, leads the typical radical character of the carbene carbon. This behaviour not only explains the carbon-centered radical-type reactivity of these complexes, but also their reduced electrophilicity (suppressing carbene-carbene dimerisation side reactions) as well as their enhanced reactivity to electron-deficient substrates. Furthermore, second coordination sphere hydrogen-bonding interactions give rise to faster reactions because H-bonds are stronger to the reduced carbene as compared to the precursor.
Such H-bonding interactions can also facilitate chirality transfer in enantioselective carbene-transfer reactions.
In order for the
Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs, leads the typical radical character of the carbene carbon. This behaviour not only explains the carbon-centered radical-type reactivity of these complexes, but also their reduced electrophilicity (suppressing carbene-carbene dimerisation side reactions) as well as their enhanced reactivity to electron-deficient substrates. Furthermore, second coordination sphere hydrogen-bonding interactions give rise to faster reactions because H-bonds are stronger to the reduced carbene as compared to the precursor.
Such H-bonding interactions can also facilitate chirality transfer in enantioselective carbene-transfer reactions.
In order for the σ bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of sy ...
to be stabilized (typically with a bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
slightly less than 1), a back-bonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic chemi ...
action from the π molecular orbital to the anti-bonding
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more no ...
π* molecular orbital is necessary and the porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
ring serves as an electron π-symmetry "buffer" to ensure this interaction is obtained.
The back-donation to the π* orbital would result in unfavorable excess electron density on the carbene carbon but the presence of adjacent functional groups (carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
or sulfonyl
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups c ...
groups have the desired electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
) relieve this electron build-up and yield the final radical electron, which occupies a single ''p'' atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
state on the carbon.
See also
References
{{reflist, 2 Carbenes Organometallic chemistry Functional groups Organic compounds