Capillary Length on:
[Wikipedia]
[Google]
[Amazon]
 The capillary length or capillary constant, is a length scaling factor that relates
The capillary length or capillary constant, is a length scaling factor that relates
 Like a droplet, bubbles are round because cohesive forces pull its molecules into the tightest possible grouping, a sphere. Due to the trapped air inside the bubble, it is impossible for the surface area to shrink to zero, hence the pressure inside the bubble is greater than outside, because if the pressures were equal, then the bubble would simply collapse. This pressure difference can be calculated from Laplace's pressure equation,
:.
For a soap bubble, there exists two boundary surfaces, internal and external, and therefore two contributions to the excess pressure and Laplace's formula doubles to
:.
The capillary length can then be worked out the same way except that the thickness of the film, must be taken into account as the bubble has a hollow center, unlike the droplet which is a solid. Instead of thinking of a droplet where each side is as in the above derivation, for a bubble is now
:,
with and the radius and thickness of the bubble respectively.
As above, the Laplace and hydrostatic pressure are equated resulting in
:.
Thus the capillary length contributes to a physiochemical limit that dictates the maximum size a soap bubble can take.
Like a droplet, bubbles are round because cohesive forces pull its molecules into the tightest possible grouping, a sphere. Due to the trapped air inside the bubble, it is impossible for the surface area to shrink to zero, hence the pressure inside the bubble is greater than outside, because if the pressures were equal, then the bubble would simply collapse. This pressure difference can be calculated from Laplace's pressure equation,
:.
For a soap bubble, there exists two boundary surfaces, internal and external, and therefore two contributions to the excess pressure and Laplace's formula doubles to
:.
The capillary length can then be worked out the same way except that the thickness of the film, must be taken into account as the bubble has a hollow center, unlike the droplet which is a solid. Instead of thinking of a droplet where each side is as in the above derivation, for a bubble is now
:,
with and the radius and thickness of the bubble respectively.
As above, the Laplace and hydrostatic pressure are equated resulting in
:.
Thus the capillary length contributes to a physiochemical limit that dictates the maximum size a soap bubble can take.
gravity
In physics, gravity () is a fundamental interaction which causes mutual attraction between all things with mass or energy. Gravity is, by far, the weakest of the four fundamental interactions, approximately 1038 times weaker than the str ...
and surface tension. It is a fundamental physical property that governs the behavior of menisci, and is found when body forces (gravity) and surface forces (Laplace pressure
The Laplace pressure is the pressure difference between the inside and the outside of a curved surface that forms the boundary between two fluid regions. The pressure difference is caused by the surface tension of the interface between liquid and ...
) are in equilibrium.
The pressure of a static fluid does not depend on the shape, total mass or surface area of the fluid. It is directly proportional to the fluid's specific weight
The specific weight, also known as the unit weight, is the weight per unit volume of a material.
A commonly used value is the specific weight of water on Earth at , which is .National Council of Examiners for Engineering and Surveying (2005). ''Fu ...
– the force exerted by gravity over a specific volume, and its vertical height. However, a fluid also experiences pressure that is induced by surface tension, commonly referred to as the Young-Laplace pressure. Surface tension originates from cohesive forces between molecules, and in the bulk
Bulk can refer to:
Industry
* Bulk cargo
* Bulk liquids
* Bulk mail
* Bulk material handling
* Bulk pack, packaged bulk materials/products
* Bulk purchasing
*
Baking
* Bulk fermentation, the period after mixing when dough is left alone to ferm ...
of the fluid, molecules experience attractive forces from all directions. The surface of a fluid is curved because exposed molecules on the surface have fewer neighboring interactions, resulting in a net force that contracts the surface. There exists a pressure difference either side of this curvature, and when this balances out the pressure due to gravity, one can rearrange to find the capillary length.
In the case of a fluid–fluid interface, for example a drop of water immersed in another liquid, the capillary length denoted or is most commonly given by the formula,
:,
where is the surface tension of the fluid interface, is the gravitational acceleration
In physics, gravitational acceleration is the acceleration of an object in free fall within a vacuum (and thus without experiencing drag (physics), drag). This is the steady gain in speed caused exclusively by the force of gravitational attract ...
and is the mass density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically ...
difference of the fluids. The capillary length is sometimes denoted in relation to the mathematical notation for curvature
In mathematics, curvature is any of several strongly related concepts in geometry. Intuitively, the curvature is the amount by which a curve deviates from being a straight line, or a surface deviates from being a plane.
For curves, the can ...
. The term capillary constant is somewhat misleading, because it is important to recognize that is a composition of variable quantities, for example the value of surface tension will vary with temperature and the density difference will change depending on the fluids involved at an interface interaction. However if these conditions are known, the capillary length can be considered a constant for any given liquid, and be used in numerous fluid mechanical problems to scale the derived equations such that they are valid for any fluid. For molecular fluids, the interfacial tensions and density differences are typically of the order of mN m−1 and g mL−1 respectively resulting in a capillary length of mm for water and air at room temperature on earth. On the other hand, the capillary length would be mm for water-air on the moon. For a soap bubble
A soap bubble is an extremely thin film of soap or detergent and water enclosing air that forms a hollow sphere with an iridescent surface. Soap bubbles usually last for only a few seconds before bursting, either on their own or on contact w ...
, the surface tension must be divided by the mean thickness, resulting in a capillary length of about meters in air! The equation for can also be found with an extra term, most often used when normalising the capillary height.
Origin
Theoretical
One way to theoretically derive the capillary length, is to imagine a liquid droplet at the point where surface tension balances gravity. Let there be a spherical droplet with radius , The characteristicLaplace pressure
The Laplace pressure is the pressure difference between the inside and the outside of a curved surface that forms the boundary between two fluid regions. The pressure difference is caused by the surface tension of the interface between liquid and ...
, due to surface tension, is equal to
:,
where is the surface tension. The pressure due to gravity (hydrostatic pressure) of a column of liquid is given by
:,
where is the droplet density, the gravitational acceleration, and is the height of the droplet.
At the point where the Laplace pressure balances out the pressure due to gravity ,
:.
Relationship with the Eötvös number
The above derivation can be used when dealing with the Eötvös number, adimensionless quantity
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
that represents the ratio between the buoyancy forces and surface tension of the liquid. Despite being introduced by Loránd Eötvös
Baron Loránd Eötvös de Vásárosnamény (or Loránd Eötvös, , '' hu, vásárosnaményi báró Eötvös Loránd Ágoston''; 27 July 1848 – 8 April 1919), also called Baron Roland von Eötvös in English literature, was a Hungarian physicis ...
in 1886, he has since become fairly dissociated with it, being replaced with Wilfrid Noel Bond such that it is now referred to as the Bond number in recent literature.
The Bond number can be written such that it includes a characteristic length- normally the radius of curvature of a liquid, and the capillary length
:,
with parameters defined above, and the radius of curvature.
Therefore the bond number can be written as
:,
with the capillary length.
If the bond number is set to 1, then the characteristic length is the capillary length
Experimental
The capillary length can also be found through the manipulation of many different physical phenomenon. One method is to focus oncapillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces li ...
, which is the attraction of a liquids surface to a surrounding solid.
Association with Jurin's law
Jurin's law
Jurin's law, or capillary rise, is the simplest analysis of capillary action—the induced motion of liquids in small channels—and states that the maximum height of a liquid in a capillary tube is inversely proportional to the tube's diameter. Ca ...
is a quantitative law that shows that the maximum height that can be achieved by a liquid in a capillary tube is inversely proportional to the diameter of the tube. The law can be illustrated mathematically during capillary uplift, which is a traditional experiment measuring the height of a liquid in a capillary tube. When a capillary tube is inserted into a liquid, the liquid will rise or fall in the tube, due to an imbalance in pressure. The characteristic height is the distance from the bottom of the meniscus to the base, and exists when the Laplace pressure and the pressure due to gravity are balanced. One can reorganize to show the capillary length as a function of surface tension and gravity.
:,
with the height of the liquid, the radius of the capillary tube, and the contact angle
The contact angle is the angle, conventionally measured through the liquid, where a liquid– vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, ...
.
The contact angle is defined as the angle formed by the intersection of the liquid-solid interface and the liquid–vapour interface. The size of the angle quantifies the wetability of liquid, i.e., the interaction between the liquid and solid surface. A contact angle of can be considered, perfect wetting.
:.
Thus the forms a cyclical 3 factor equation with .
This property is usually used by physicists to estimate the height a liquid will rise in a particular capillary tube, radius known, without the need for an experiment. When the characteristic height of the liquid is sufficiently less than the capillary length, then the effect of hydrostatic pressure due to gravity can be neglected.
Using the same premises of capillary rise, one can find the capillary length as a function of the volume increase, and wetting perimeter of the capillary walls.
Association with a sessile droplet
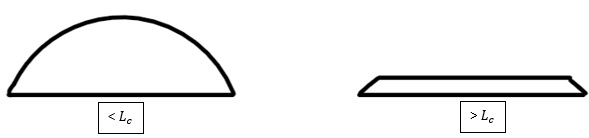
Another way to find the capillary length is using different pressure points inside a sessile droplet, with each point having a radius of curvature, and equate them to the Laplace pressure equation. This time the equation is solved for the height of the meniscus level which again can be used to give the capillary length. The shape of a sessile droplet is directly proportional to whether the radius is greater than or less than the capillary length. Microdrops are droplets with radius smaller than the capillary length, and their shape is governed solely by surface tension, forming a spherical cap shape. If a droplet has a radius larger than the capillary length, they are known as macrodrops and the gravitational forces will dominate. Macrodrops will be 'flattened' by gravity and the height of the droplet will be reduced.
History
The investigations in capillarity stem back as far asLeonardo da Vinci
Leonardo di ser Piero da Vinci (15 April 14522 May 1519) was an Italian polymath of the High Renaissance who was active as a painter, Drawing, draughtsman, engineer, scientist, theorist, sculptor, and architect. While his fame initially re ...
, however the idea of capillary length was not developed until much later. Fundamentally the capillary length is a product of the work of Thomas Young and Pierre Laplace
Pierre-Simon, marquis de Laplace (; ; 23 March 1749 – 5 March 1827) was a French scholar and polymath whose work was important to the development of engineering, mathematics, statistics, physics, astronomy, and philosophy. He summarized ...
. They both appreciated that surface tension arose from cohesive forces between particles and that the shape of a liquid's surface reflected the short range of these forces. At the turn of the 19th century they independently derived pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
equations, but due to notation and presentation, Laplace often gets the credit. The equation showed that the pressure within a curved surface between two static fluids is always greater than that outside of a curved surface, but the pressure will decrease to zero as the radius approached infinity. Since the force is perpendicular to the surface and acts towards the centre of the curvature, a liquid will rise when the surface is concave and depress when convex. This was a mathematical explanation of the work published by James Jurin
James Jurin FRS FRCP (baptised 15 December 168429 March 1750) was an English scientist and physician, particularly remembered for his early work in capillary action and in the epidemiology of smallpox vaccination. He was a staunch proponent o ...
in 1719, where he quantified a relationship between the maximum height taken by a liquid in a capillary tube and its diameter – Jurin's law
Jurin's law, or capillary rise, is the simplest analysis of capillary action—the induced motion of liquids in small channels—and states that the maximum height of a liquid in a capillary tube is inversely proportional to the tube's diameter. Ca ...
. The capillary length evolved from the use of the Laplace pressure equation at the point it balanced the pressure due to gravity, and is sometimes called the ''Laplace capillary constant,'' after being introduced by Laplace in 1806.
In nature
Bubbles
See also
*Capillarity
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces li ...
* Surface tension
* Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
* Bond number
* Jurin's law
Jurin's law, or capillary rise, is the simplest analysis of capillary action—the induced motion of liquids in small channels—and states that the maximum height of a liquid in a capillary tube is inversely proportional to the tube's diameter. Ca ...
* Young–Laplace equation
In physics, the Young–Laplace equation () is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or ...
References
{{reflist Fluid dynamics