Bredt's rule on:
[Wikipedia]
[Google]
[Amazon]
Bredt's rule is an empirical observation in 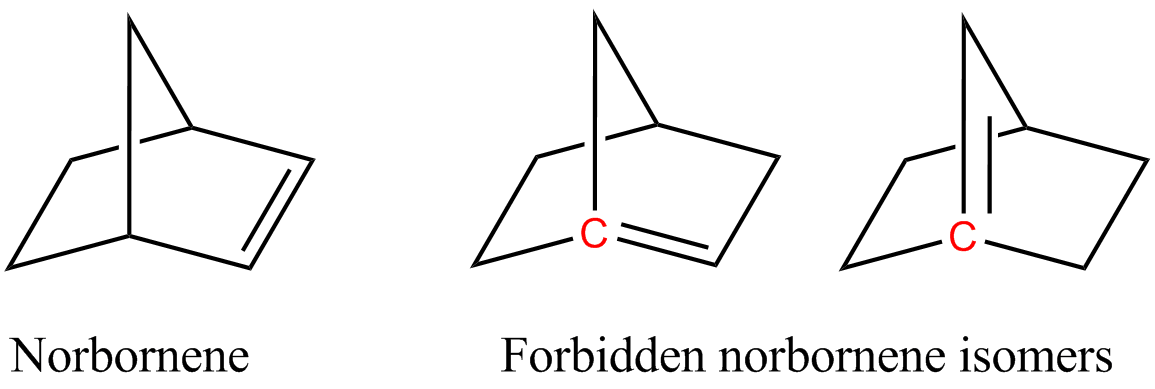 In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a
In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
that states that a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds.
For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare:
 In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a
In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a trans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Arts, entertainment, and media
* Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom
* ''Trans'' (fil ...
double bond on a ring, which is not stable for small rings (fewer than eight atoms) due to a combination of ring strain, and angle strain (nonplanar alkene). The p orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
of the bridgehead atom and adjacent atoms are orthogonal and thus are not aligned properly for the formation of pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s. Fawcett quantified the rule by defining ''S'' as the number of non-bridgehead atoms in a ring system, and postulated that stability required ''S'' ≥ 9 in bicyclic systems and ''S'' ≥ 11 in tricyclic systems. There has been an active research program to seek compounds inconsistent with the rule, and for bicyclic systems a limit of S ≥ 7 is now established with several such compounds having been prepared. The above norbornene system has ''S'' = 5 and so they are not preparable.
Bredt's rule can be useful for predicting which isomer is obtained from an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
in a bridged ring system. It can also be applied to reaction mechanisms that go via carbocations and, to a lesser degree, via free radicals, because these intermediates, like carbon atoms involved in a double bond, prefer to have a planar geometry with 120 degree angles and sp2 hybridization. The rule also allows the rationalisation of observations. For example, bicyclo .3.1ndecane-11-one-1-carboxylic acid undergoes decarboxylation on heating to 132 °C, but the similar compound bicyclo .2.1eptan-7-one-1-carboxylic acid remains stable beyond 500 °C, despite both being β-keto acids with the carbonyl group on a one-carbon bridge and the carboxylate group on the bridgehead. The mechanism of decarboxylation involves an enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
intermediate, which is an ''S'' = 9 species in the former case and an ''S'' = 5 species in the latter, preventing the decarboxylation in the smaller ring system.
An anti-bredt molecule is one that is found to exist and be stable (within certain parameters) despite this rule. A recent (2006) example of such a molecule is 2-quinuclidonium tetrafluoroborate. Bridgehead double bonds can be found in some natural products, discussed in a review by Mak, Pouwer and Williams, and an older review by Shea looked at bridgehead alkenes more generally.
References
{{Authority control Physical organic chemistry Stereochemistry