Brookhart's Acid on:
[Wikipedia]
[Google]
[Amazon]
Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis ,5-bis(trifluoromethyl)phenylorate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.
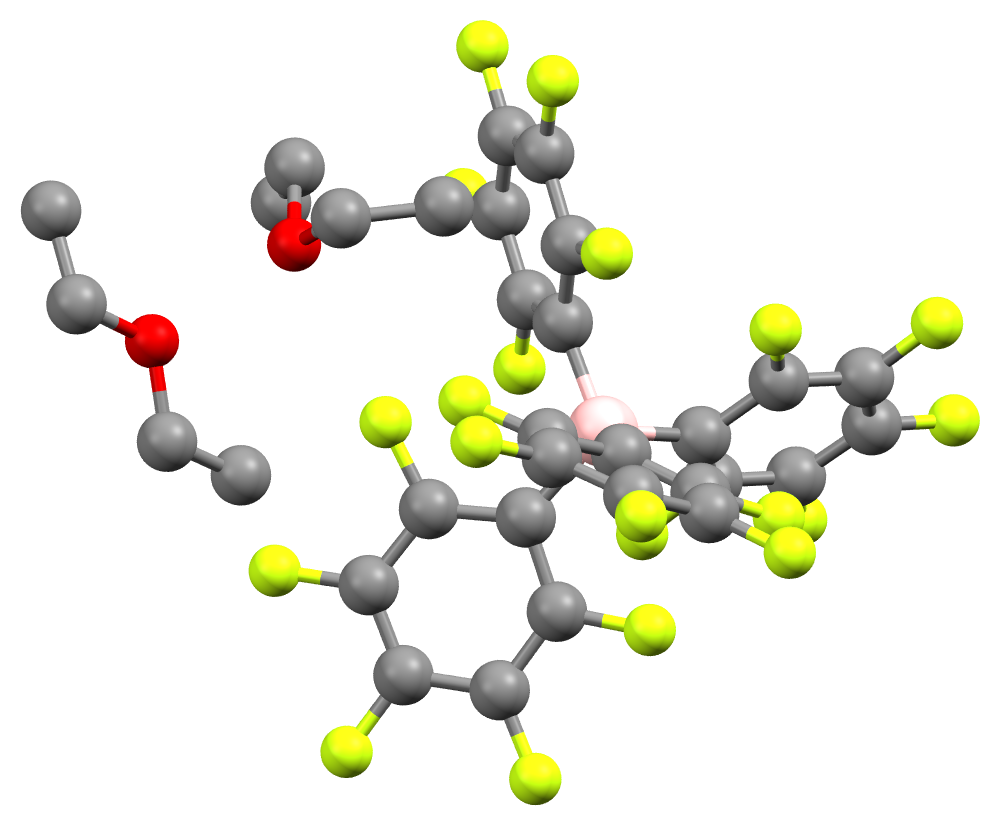 The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3.
(OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by both ethereal oxygen centers, although the crystal was not good enough to determine whether the proton is located symmetrically or unsymmetrically between the two.Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H. G. (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid (OEt2)2sup>+B(C6F5)4]−". Organometallics vol. 19, p. 1442.
The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3.
(OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by both ethereal oxygen centers, although the crystal was not good enough to determine whether the proton is located symmetrically or unsymmetrically between the two.Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H. G. (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid (OEt2)2sup>+B(C6F5)4]−". Organometallics vol. 19, p. 1442.
 Polyketones, thermoplastic polymers, are formed by the copolymerisation of carbon monoxide and one or more alkenes (typically
Polyketones, thermoplastic polymers, are formed by the copolymerisation of carbon monoxide and one or more alkenes (typically
Preparation
This compound is prepared by treatment of NaBAr′4 in diethyl ether (Et2O) with hydrogen chloride: : NaBAr′4 + HCl + 2 Et2O → (OEt2)2sup>+ + NaCl NaBAr′4 is soluble in diethyl ether, whereassodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
is not. Precipitation of sodium chloride thus drives the formation of the oxonium acid compound, which is isolable as a solid.
Structure and properties
 The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3.
(OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by both ethereal oxygen centers, although the crystal was not good enough to determine whether the proton is located symmetrically or unsymmetrically between the two.Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H. G. (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid (OEt2)2sup>+B(C6F5)4]−". Organometallics vol. 19, p. 1442.
The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3.
(OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by both ethereal oxygen centers, although the crystal was not good enough to determine whether the proton is located symmetrically or unsymmetrically between the two.Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H. G. (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid (OEt2)2sup>+B(C6F5)4]−". Organometallics vol. 19, p. 1442.
Uses
Traditionalweakly coordinating anions
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly fou ...
, such as perchlorate, tetrafluoroborate, and hexafluorophosphate, will nonetheless coordinate to very electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
cations, making these counterions unsuitable for some complexes. The highly reactive species p2Zr(CH3)sup>+, for example, has been reported to abstract F− from PF6. Starting in the 1980s, new types of weakly coordinating anions began to be developed. BAr′4 anions are used as counterions for highly electrophilic, cationic transition metal species, as they are very weakly coordinating and unreactive towards electrophilic attack. One common method of generating these cationic species is via protonolysis of a dialkyl complexes or an olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
complex. For example, an electrophilic palladium catalyst, CH3CN)">acetonitrile.html" ;"title="2,2′-bipyridine)Pd(CH3)(acetonitrile">CH3CN)BAr′4], is prepared by protonating the dimethyl complex with Brookhart's acid. This electrophilic, cationic palladium species is used for the polymerization of olefins with carbon monoxide to polyketones in aprotic solvents.
Potential application
ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
with propylene). The process utilises a palladium(II) catalyst with a bidentate ligand like 2,2′-bipyridine or 1,10-phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviated ...
(phen) with a non-coordinating BARF counterion, such as phen)Pd(CH3)(CO)ArF4. The preparation of the catalyst involves the reaction of a dimethyl palladium complex with Brookhart's acid in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
with loss of methane and the catalytic species is formed by uptake of carbon monoxide to displace acetonitrile.
: Et2O)2HArF4 + phen)Pd(CH3)2 + MeCN → phen)Pd(CH3)(MeCN)ArF4 + 2 Et2O + CH4
: phen)Pd(CH3)(MeCN)ArF4 + CO → phen)Pd(CH3)(CO)ArF4 + MeCN
The mechanism involves migratory insertion whereby the polymer chain is bound to the catalytic centre and grows by the sequential insertion of carbon monoxide and the alkene between the palladium atom and the existing chain. Defects occur when insertions do not alternate – that is, a carbon monoxide insertion follows a carbon monoxide insertion or an alkene insertion follows an alkene insertion – these are highlighted in red in the figure below. This catalyst produces a very low rate of defects due to the difference in Gibbs energy of activation of each insertion – the energy barrier to inserting an alkene immediately following an alkene insertion is ~12 kJ mol−1 higher than barrier to carbon monoxide insertion. Use of monodentate phosphine ligands also leads to undesirable side-products but bidentate phosphine ligands like 1,3-bis(diphenylphosphino)propane have been used industrially.
References
{{Reflist Acids Non-coordinating anions Trifluoromethyl compounds Oxonium compounds