Boudouard Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Boudouard reaction, named after 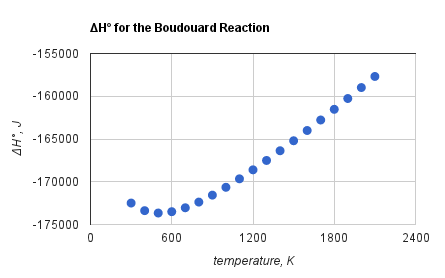 The Boudouard reaction to form carbon dioxide and carbon is
The Boudouard reaction to form carbon dioxide and carbon is
/ref> as shown to the side. While the formation enthalpy of is higher than that of , the formation entropy is much lower. Consequently, the standard free energy of formation of from its component elements is almost constant and independent of the temperature, while the free energy of formation of decreases with temperature. At high temperatures, the forward reaction becomes
''Furnace atmospheres and carbon control''
Metals Park, OH 964 carbon monoxide is the stable oxide of carbon. When a gas rich in is cooled to the point where the activity of carbon exceeds one, the Boudouard reaction can take place. Carbon monoxide then tends to disproportionate into carbon dioxide and graphite, which forms
Octave Leopold Boudouard
Octave Leopold Boudouard (1872–1923) was a French chemist known for his 1905 discovery of the Boudouard reaction.
Career
Octave Leopold Boudouard became a professor at the Conservatoire National des Arts et Métiers
A music school is an edu ...
, is the redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reaction of a chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the ...
mixture of carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
at a given temperature. It is the disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term ca ...
of carbon monoxide into carbon dioxide and graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
or its reverse:
::2CO + C
 The Boudouard reaction to form carbon dioxide and carbon is
The Boudouard reaction to form carbon dioxide and carbon is exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
at all temperatures. However, the standard enthalpy of the Boudouard reaction becomes less negative with increasing temperature,Reaction Web/ref> as shown to the side. While the formation enthalpy of is higher than that of , the formation entropy is much lower. Consequently, the standard free energy of formation of from its component elements is almost constant and independent of the temperature, while the free energy of formation of decreases with temperature. At high temperatures, the forward reaction becomes
endergonic
In chemical thermodynamics, an endergonic reaction (; also called a heat absorbing nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and an additional driving fo ...
, favoring the (exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs f ...
) reverse reaction toward CO, even though the forward reaction is still exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
.
The effect of temperature on the extent of the Boudouard reaction is indicated better by the value of the equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
than by the standard free energy of reaction. The value of log10(''K''eq) for the reaction as a function of temperature in Kelvin (valid between 500–) is approximately:
:
has a value of zero at .
The implication of the change in ''K''eq with temperature is that a gas containing may form elemental carbon if the mixture cools below a certain temperature. The thermodynamic activity of carbon may be calculated for a / mixture by knowing the partial pressure of each species and the value of ''K''eq. For instance, in a high temperature reducing environment, such as that created for the reduction of iron oxide in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric p ...
or the preparation of carburizing
Carburising, carburizing (chiefly American English), or carburisation is a heat treatment process in which iron or steel absorbs carbon while the metal is heated in the presence of a carbon-bearing material, such as charcoal or carbon monoxid ...
atmospheres,ASM Committee on Furnace Atmospheres''Furnace atmospheres and carbon control''
Metals Park, OH 964 carbon monoxide is the stable oxide of carbon. When a gas rich in is cooled to the point where the activity of carbon exceeds one, the Boudouard reaction can take place. Carbon monoxide then tends to disproportionate into carbon dioxide and graphite, which forms
soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyse ...
.
In industrial catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
, this is not just an eyesore; sooting (also called coking) can cause serious and even irreversible damage to catalysts and catalyst beds. This is a problem in the catalytic reforming of petroleum and the steam reforming of natural gas.
The reaction is named after the French chemist, Octave Leopold Boudouard
Octave Leopold Boudouard (1872–1923) was a French chemist known for his 1905 discovery of the Boudouard reaction.
Career
Octave Leopold Boudouard became a professor at the Conservatoire National des Arts et Métiers
A music school is an edu ...
(1872–1923), who investigated this equilibrium in 1905.
Uses
Although the damaging effect ofcarbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
on catalysts is undesirable, this reaction has been used in producing graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
flakes, filamentous graphite and lamellar graphite crystallites, as well as producing carbon nanotubes
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon nan ...
. In graphite production, catalysts used are molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with le ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
, while in carbon nanotube production, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with le ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and Ni-MgO catalysts are used.
The Boudouard reaction is an important process inside a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric p ...
. The reduction of iron oxides is not achieved by carbon directly, as reactions between solids are typically very slow, but by carbon monoxide. The resulting carbon dioxide undergoes a (reverse) Boudouard reaction upon contact with coke carbon.
Undesired occurrence
While the Boudouard reaction is used deliberately in some processes, it is undesired in others. In the gas cooled, graphite moderated British nuclear reactors (Magnox
Magnox is a type of nuclear power/production reactor that was designed to run on natural uranium with graphite as the moderator and carbon dioxide gas as the heat exchange coolant. It belongs to the wider class of gas-cooled reactors. The na ...
and AGR) reaction between the CO2 coolant and the graphite moderator had to be avoided or at least kept to a minimum. As the equilibrium of the reaction shifts in favor of carbon at lower temperatures, this was solved in the Magnox reactor by simply having a lower operating temperature. However, this in turn reduced the achievable thermal efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For a ...
. In the AGR, which was supposed to improve upon the lessons learned from the Magnox, a higher coolant outlet temperature was an explicit design goal (Britain being reliant on coal power at the time, the aim was to achieve the same steam temperature as in coal fired plants) and thus a re-entrant flow of coolant at the lower boiler outlet temperature of is utilized to cool the graphite, ensuring that the graphite core temperatures do not vary too much from those seen in a Magnox reactor.
References
External links
{{cite web , last = Robinson , first = R. J. , authorlink = , title = Boudouard Process for Synthesis Gas , website = , publisher = ABC of Alternative Energy , url = http://www.abc-alternative-energy.de/bioenergy/boudouard-process.html , accessdate = 12 July 2013 , archive-date = 21 January 2018 , archive-url = https://web.archive.org/web/20180121072336/http://www.abc-alternative-energy.de/bioenergy/boudouard-process.html , url-status = dead Carbon-heteroatom bond forming reactions Name reactions Gas technologies