bonding orbital on:
[Wikipedia]
[Google]
[Amazon]
In
 In the classic example of the H2 MO, the two separate H atoms have identical atomic orbitals. When creating the molecule dihydrogen, the individual valence orbitals, 1''s'', either: merge in phase to get bonding orbitals, where the
In the classic example of the H2 MO, the two separate H atoms have identical atomic orbitals. When creating the molecule dihydrogen, the individual valence orbitals, 1''s'', either: merge in phase to get bonding orbitals, where the  When looking at helium, the atom holds two electrons in each valence 1''s'' shell. When the two atomic orbitals come together, they first fill in the bonding orbital with two electrons, but unlike hydrogen, it has two electrons left, which must then go to the antibonding orbital. The instability of the antibonding orbital cancels out the stabilizing effect provided by the bonding orbital; therefore, dihelium's
When looking at helium, the atom holds two electrons in each valence 1''s'' shell. When the two atomic orbitals come together, they first fill in the bonding orbital with two electrons, but unlike hydrogen, it has two electrons left, which must then go to the antibonding orbital. The instability of the antibonding orbital cancels out the stabilizing effect provided by the bonding orbital; therefore, dihelium's
 An example of a MO of a simple conjugated π system is butadiene. To create the MO for
An example of a MO of a simple conjugated π system is butadiene. To create the MO for
 The spherical 3D shape of ''s'' orbitals have no directionality in space and ''px'', ''py'', and ''pz'' orbitals are all 90o with respect to each other. Therefore, in order to obtain orbitals corresponding to
The spherical 3D shape of ''s'' orbitals have no directionality in space and ''px'', ''py'', and ''pz'' orbitals are all 90o with respect to each other. Therefore, in order to obtain orbitals corresponding to
theoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface o ...
, the bonding orbital is used in molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
(MO) theory to describe the attractive interactions between the atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s of two or more atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s in a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
. In MO theory, electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s are portrayed to move in wave
In physics, mathematics, and related fields, a wave is a propagating dynamic disturbance (change from equilibrium) of one or more quantities. Waves can be periodic, in which case those quantities oscillate repeatedly about an equilibrium (res ...
s. When more than one of these waves come close together, the in-phase
In physics and mathematics, the phase of a periodic function F of some real variable t (such as time) is an angle-like quantity representing the fraction of the cycle covered up to t. It is denoted \phi(t) and expressed in such a scale that it v ...
combination of these waves produces an interaction that leads to a species that is greatly stabilized. The result of the waves’ constructive interference
In physics, interference is a phenomenon in which two waves combine by adding their displacement together at every single point in space and time, to form a resultant wave of greater, lower, or the same amplitude. Constructive and destructive ...
causes the density of the electrons to be found within the binding region, creating a stable bond between the two species.
Diatomic molecules
 In the classic example of the H2 MO, the two separate H atoms have identical atomic orbitals. When creating the molecule dihydrogen, the individual valence orbitals, 1''s'', either: merge in phase to get bonding orbitals, where the
In the classic example of the H2 MO, the two separate H atoms have identical atomic orbitals. When creating the molecule dihydrogen, the individual valence orbitals, 1''s'', either: merge in phase to get bonding orbitals, where the electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
is in between the nuclei of the atoms; or, merge out of phase to get antibonding orbitals, where the electron density is everywhere around the atom except for the space between the nuclei of the two atoms. Bonding orbitals lead to a more stable species than when the two hydrogens are monatomic. Antibonding orbitals are less stable because, with very little to no electron density in the middle, the two nuclei (holding the same charge) repulse each other. Therefore, it would require more energy to hold the two atoms together through the antibonding orbital. Each electron in the valence 1''s'' shell of hydrogen come together to fill in the stabilizing bonding orbital. So, hydrogen prefers to exist as a diatomic, and not monatomic, molecule.
 When looking at helium, the atom holds two electrons in each valence 1''s'' shell. When the two atomic orbitals come together, they first fill in the bonding orbital with two electrons, but unlike hydrogen, it has two electrons left, which must then go to the antibonding orbital. The instability of the antibonding orbital cancels out the stabilizing effect provided by the bonding orbital; therefore, dihelium's
When looking at helium, the atom holds two electrons in each valence 1''s'' shell. When the two atomic orbitals come together, they first fill in the bonding orbital with two electrons, but unlike hydrogen, it has two electrons left, which must then go to the antibonding orbital. The instability of the antibonding orbital cancels out the stabilizing effect provided by the bonding orbital; therefore, dihelium's bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
is 0. This is why helium would prefer to be monatomic over diatomic.
Polyatomic molecules

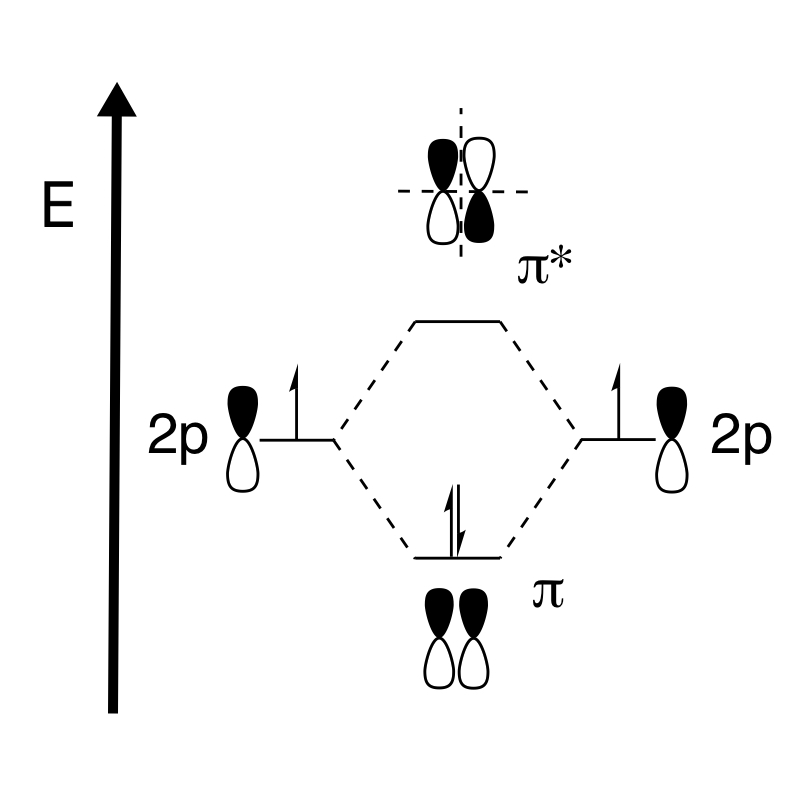
Bonding MOs of pi bonds
Pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s are created by the “side-on” interactions of the orbitals. Once again, in molecular orbitals, bonding pi (π) electrons occur when the interaction of the two π atomic orbitals are in-phase. In this case, the electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
of the π orbitals needs to be symmetric along the mirror plane in order to create the bonding interaction. Asymmetry along the mirror plane will lead to a node in that plane and is described in the antibonding orbital, π*.
 An example of a MO of a simple conjugated π system is butadiene. To create the MO for
An example of a MO of a simple conjugated π system is butadiene. To create the MO for butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
, the resulting π and π* orbitals of the previously described system will interact with each other. This mixing will result in the creation of 4 group orbitals (which can also be used to describe the π MO of any diene): π1 contains no vertical nodes
In general, a node is a localized swelling (a "knot") or a point of intersection (a Vertex (graph theory), vertex).
Node may refer to:
In mathematics
*Vertex (graph theory), a vertex in a mathematical graph
*Vertex (geometry), a point where two ...
, π2 contains one and both are considered bonding orbitals; π3 contains 2 vertical nodes, π4 contains 3 and are both considered antibonding orbitals.
Localized molecular orbitals
 The spherical 3D shape of ''s'' orbitals have no directionality in space and ''px'', ''py'', and ''pz'' orbitals are all 90o with respect to each other. Therefore, in order to obtain orbitals corresponding to
The spherical 3D shape of ''s'' orbitals have no directionality in space and ''px'', ''py'', and ''pz'' orbitals are all 90o with respect to each other. Therefore, in order to obtain orbitals corresponding to chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s to describe chemical reactions, Edmiston and Ruedenberg pioneered the development of localization procedures. For example, in CH4, the four electrons from the 1''s'' orbitals of the hydrogen atoms and the valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s from the carbon atom (2 in ''s'' and 2 in ''p'') occupy the bonding molecular orbitals, σ and π. The delocalized MOs of the carbon atom in the molecule of methane can then be localized to give four ''sp''3 hybrid orbitals
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
.
Applications
Molecular orbitals and, more specifically, the bonding orbital is a theory that is taught in all different areas of chemistry, from organic to physical and even analytical, because it is widely applicable. Organic chemists use molecular orbital theory in their thought rationale for reactions; analytical chemists use it in different spectroscopy methods; physical chemists use it in calculations; it is even seen in materials chemistry throughband theory
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have (called ''band gaps'' or '' ...
—an extension of molecular orbital theory.
References